A novel apoptosis research method with imaging-combined flow cytometer and HITC or IR-125 staining
Abstract
The most commonly used methods for apoptotic research include terminal transferase-mediated dUTP nick end-labeling, annexin V testing of phosphatidylserine translocation from the inner leaflet to the outer plasma membrane by flow cytometry, DNA electrophoresis, and cell morphology. These methods provide apoptotic information from different aspects. To find a new way in apoptosis research and potential clinical application, we recently developed a novel method with an imaging-combined flow cytometer (IFC) and an innovative cell staining process by using 2-[7-(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)-1,3,5-heptatrienyl]-1,3,3-trimethyl-3H-indolium iodide (HITC) and 2-[7-[1,3-dihydro-1,1-dimethyl-3-(4-sulfobutyl)-2H-benz[e]indol-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-1H-benz[e]indolium hydroxide, inner salt, sodium salt (IR-125). The IFC used in the research is a new generation of cytometry designed for simultaneous observations of cell populations and images. This is possible because the IFC is equipped with dual laser beams, one argon and one infrared. A promyelocytic leukemia cell line, HL-60, was used in the research. The cells were stained with our newly developed HITC or IR-125 staining method and a traditional nucleic acid dye, propidium iodide. The cells stained with HITC or IR-125 appeared completely dark in the IFC image window before washing. Phosphate buffered saline wash did not change the cell appearance. A wash with 50% methanol caused the cells to have a clear cell image with bright nuclei on the IFC. To obtain apoptotic cells, we treated the HL-60 cells with 0.15 μM of camptothecin (CAM), a topoisomerase I inhibitor and experimental apoptosis inducer, for 4 h. The control showed larger round cells with bright nuclei and one to three dark nucleoli. The CAM-induced apoptotic cells were smaller, with fragmented and condensed nuclei on the IFC. These appearances were identical to the cell morphology of with light and electron microscopy. We used other methods including FACScan and DNA electrophoresis to confirm the apoptotic changes after CAM treatment and compared them with the IFC method. In addition, we found that the novel method with the IFC and HITC or IR-125 staining can show not only cell apoptotic changes but also peripheral blood cell populations and images simultaneously. This study suggests many potential applications of the IFC and this novel staining method in other cellular biological researches and clinical assays. Cytometry (Clin. Cytometry) 50:267–274, 2002. © 2002 Wiley-Liss, Inc.
Development and applications of flow cytometry have been well reviewed (1). Because it is considered a powerful method when performing quantitative cell study, flow cytometry has been used in clinical assay and biomedical research including the study of cell surface antigens and cell cycles (1-3). However, traditional cytometry provides information only about cell populations; cell morphology or images cannot be observed simultaneously. Several image cytometers have been used in biomedical research (4-6). They were designed based on the microscope and have the function of providing cell population analysis. These image cytometers can provide cell morphology or cell images and show cell populations from cultured or cytospun slides; however, the cells shown cannot be in suspension.
The imaging combined flow cytometer (IFC), a new generation of image cytometry, is being tested in different fields for its applicability. Currently, the IFC is equipped with a 480-nm wavelength argon laser in the cytometric component to quantitate cell populations and a 780-nm wavelength infrared laser on its imaging component to observe cell morphology. It can be used to observe cells in solution and directly in blood. The main advantage of the IFC is that it can be used to observe cell populations and morphology or images simultaneously. One can use it to study cell images in different cell populations in a single operation.
Even though there is some interest in using IFC in biomedical research and clinical observation, only a few research studies have been conducted. Auramine O is the only dye that has been used on the IFC. Auramine O is a fluorescent dye with an excitation wavelength of 435 nm and an emission of 550 nm. It is useful for observing cell population with an argon laser beam (7), but useless in image studies because these wavelengths are not in the range of the current IFC image laser beam.
Apoptosis is a word derived from the Greek apo, meaning “off,” and ptosis, meaning “fall.” Thus, it refers to a “falling off,” in this case, of cellular viability (8). It is an active process of gene-directed cellular self-destruction and has been described as programmed cell death, although the distinction has been made between unscheduled cell death and apoptosis. The hallmark for identification of apoptosis is fragmentation of the cell's DNA via endonucleases (8-12). Because of the significance of apoptosis in physiology, pathophysiology (especially in cancer, acquired immunodeficiency syndrome, autoimmune diseases, and degenerative diseases of the central nervous system), and pharmacology, apoptosis has been studied broadly in different fields. We selected apoptotic cells as the model in the initial IFC study because of their critical place in basic and clinical medicine and the apparent changes of cell population and morphology, especially with regard to nuclei and cell size. In this article, we report the development of novel staining methods on the IFC, the treatment of cultured HL-60 cells to obtain apoptotic cells, and observations of apoptotic and control cells with IFC and other methods such as light and electron microscopy, DNA electrophoresis, and FACScan.
MATERIAL AND MTHODS
Cell Culture and Treatment
HL-60 cells, a promyelocytic leukemia cell line from ATCC (Rockville, MD), were cultured in RPMI 1640 with 15% fetal bovine serum and penicillin/streptomycin in a humidity incubator with 5% CO2 at 37°C. HL-60 cells were passed 1 day before treatment. The cells were counted to make sure there were fewer than 1 × 106/ml and treated with camptothecin (CAM), a topoisomerase I inhibitor and an experimental apoptosis inducer (13-16). CAM was obtained from Sigma (St. Louis, MO; catalogue number C9911). The final concentration of CAM was 0.15 μM. HL-60 cells were treated for 4 h. The treated and control cells were used for apoptotic analysis with IFC, FACScan, cell morphology, and DNA electrophoresis.
Cell Staining for IFC
To stain the cells properly for the IFC study, we developed a novel staining method by using 2-[7-(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)-1,3,5-heptatrienyl]-1,3,3-trimethyl-3H-indolium iodide (HITC) or 2-[7-[1,3-dihydro-1,1-dimethyl-3-(4-sulfobutyl)-2H-benz[e]indol-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-1H-benz[e]indolium hydroxide, inner salt, sodium salt (IR-125). The dyes were dissolved in methanol with a concentration of 0.4% as 10× stock solution and kept at 4°C until used. A sample of 106 cultured HL-60 cells was centrifuged at 300g for 5 min and washed once with phosphate buffered saline (PBS). The washed cells were resuspended in 1 ml of PBS and then mixed with 2 ml of methanol by using a gentle vortex. The cells were fixed on ice for 30 min, centrifuged at 300g for 5 min, resuspended in 3 ml of PBS with 0.1% RNase, kept at room temperature for 30 min, resuspended in PBS, and mixed with 0.4% HITC or IR-125 in methanol to make a final dye concentration of 0.04%. Cells were stained for 30 min, washed with PBS once, and incubated with 50% methanol for 10 min. After washing, propidium iodide (PI) was added to a final concentration of 50 μg/ml, and the sample was stained for 30 min. PI staining was accompanied by RNase treatment in some experiments. Cells with different treatments and at different staining stages were observed on the IFC.
Cell Morphology
CAM-treated and control cells were cytospun on slides with Cytospin3 (Shandon, Pittsburgh, PA) and stained with an automated slide stainer, the Bayer Hematek 2000 (Bayer, Elkhart, IN), with Wright-Giemsa staining. The cells were observed with a Leitz Diaplan microscope. Images were collected with a ProgRes 3102 digital video camera and Roche Image Manager 2.2.2. The cells also were observed by using a routine method on the Philips CMIO electron microscope.
DNA Electrophoresis
For DNA electrophoresis, 5 × 106 cells were washed once with PBS, mixed in 1× TBE buffer (Trisbase, Boric Acid and EDTA) containing 0.25% Nonident P-40 and 0.1 mg/ml of RNase, and were incubated at 37°C for 30 min. Cells were then incubated another 30 min at 37°C with newly added proteinase K (1 mg/ml). After the incubation, 0.1 ml of 6× electrophoresis loading buffer was added and mixed. Twenty-five microliters from each tube was loaded into one well. One well was loaded with a standard marker (100–2,072 base pairs). The gels were stained with ethidium bromide. The samples were run on 1.5% agarose (Gibco, grand Island, NY; catalogue number 15510-019) gel at 60 V.
FACScan Analysis
Forward scatter and fluorescent intensity of PI staining were determined by flow cytometry with the use of a FACScan (Becton Dickinson Iinstruments, San Jose, CA) equipped with an argon–ion excitation laser (488 nm). Fluorescent signals were collected at a 90-degree angle to the light beam, split by a dichroic mirror, and detected by a photomultiplier tube.
IFC Analysis
The IFC is an experimental prototype developed by Sysmex Corporation, Ltd. (Kobe, Japan). The IFC is equipped with a 480-nm wavelength argon laser in the cytometric section to count cell population and a 780-nm wavelength infrared laser in the image section to observe cell morphology and image simultaneously. The imaging device also consists of an optical fiber, a condenser lens, an objective lens (40×, numerical aperture of 0.55), a relay lens (2× or 0.5×), an XC-73 CCD camera with a pixel size of 6.35 × 7.4 μm (horizonal × vertical; Sony, Tokyo, Japan), and an image processing unit. The optical beam of the pulse laser is focused 12 μm downstream from the point of focus of the argon–ion laser. The pulse laser is used with a fiber coupler, an optical fiber, and a condenser lens as the partial coherent light source for imaging. The optical fiber provides an optical nonlinear effect to decrease coherency and enhance the optical uniformity at the point of focus. Objective lenses can be selected for high (80×) or low (20×) magnification by combination of the relay and objective lenses according to the target cells. In this research, all cell images were observed with an objective lens with 80× magnification. The cell sheath flow buffer used in the experiment was RET-SEARCH™ from Sysmex Corporation. Cell image (stained with HITC or IR-125) and cell populations (stained with PI) were detected when each cell passed through the laser beam. The collected data were stored in the memory of the image process unit with ordinal flow cytometric parameters such as forward light scatter and fluorescence intensities. These permit the identification of the morphology of a cell that is shown as a corresponding dot on the scattergram. On the IFC control panel, there are some buttons to change the indicator adjustment. They are FFL (forward fluorescent light; includes all fluorescent light >530 nm), GFL (green fluorescent light; wavelengths of 515–545 nm), OFL (orange fluorescent light; >650 nm), RFL (red fluorescent light; 564–606 nm), and SSC (side scattergram). The FFL is derived from forward scattered light, and the other three are derived from side scattered light. All parameters, image focusing, and image position are adjustable so as to obtain the best measurements for different samples.
RESULTS
Cell Staining With HITC and IR-125
The new staining method was based on the wavelength of the image laser beam on the IFC. The dyes, HITC and IR-125, were tested for cell staining. After staining the HL-60 cells with the method described in Materials and Methods, the entire cell became dark in the IFC image window due to the dyes' light absorption properties (Fig. 1). PBS washing did not change cell behavior or appearance. After washing the cells for 10 min with 50% methanol, the cell nucleus became bright, and FFL and OFL on the IFC increased significantly (Figs. 1, 2).
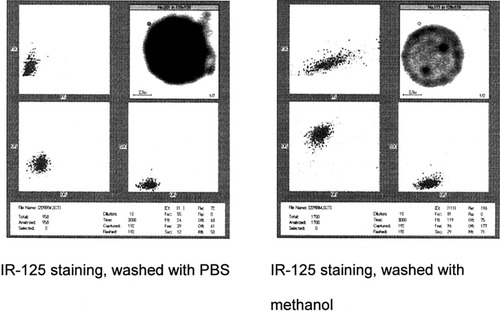
Dot plots and cell image of HL-60 cells stained with IR-125. Left: The cell image and cell populations did not change after washing with PBS. Right: The cell image showed clear contrast among the nucleus, nucleoli, and cytoplasm, and the fluorescent intensity increased significantly after washing with 50% methanol.
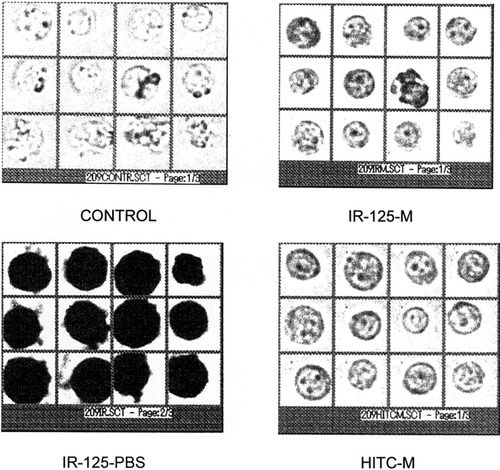
Images of multicell panels with different staining methods and at different washing stages. Control cells have no staining. IR-125–PBS was stained with IR-125 and washed with PBS. IR-125–M and HITC–M were stained with IR-125 or HITC, respectively, and washed with 50% methanol.
Cell Morphology of Apoptotic Cells
Light microscopy.
HL-60 cells were treated with 0.15 μM of CAM for 4 h. This treatment is a typical method to induce HL-60 cell apoptosis. After treatment, control and treated cells were stained by the Wright-Giemsa method. CAM-treated cells showed typical nuclear fragmentation. The cells with fragmented nuclei had condensed nuclear pieces and pinkish cytoplasm (Fig. 3).
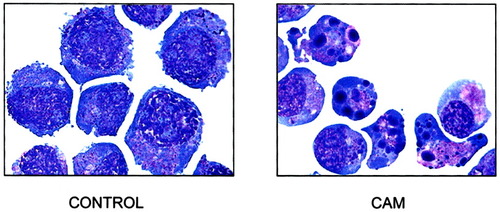
Light microscope cell morphology of control cells (left) and apoptotic cells induced by CAM (right). Control cells showed larger, round nuclei and evenly distributed cytoplasm. The CAM-treated cells showed fragmented and condensed nuclei, with pinkish and shrunken cytoplasm.
Electron microscopy.
Electron microscopy showed that control cells had the typical HL-60 cell morphology of large, round, regular nuclei and nucleoli. Apoptotic cells treated with CAM had fragmented and condensed nuclei, were smaller, and had some common apoptotic morphology changes such as apoptotic bodies, crescentic cap, and nucleus-derived vesicles (Fig. 4).
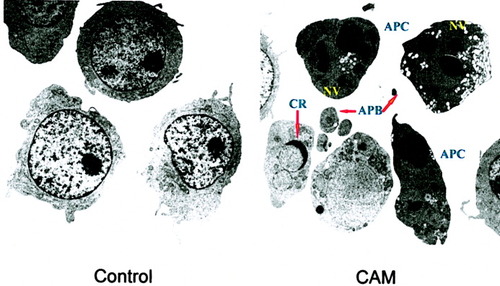
Control and CAM-treated cells under the electron microscope. CAM-treated cells are smaller, with fragmented and condensed nuclei. APC, apoptotic cells; APB, apoptotic body; CR, crescentic cap; NV, nucleus-derived vesicle. All are apoptotic changes. The control cells are larger, with regular nuclei and nucleoli.
DNA Electrophoresis of Apoptotic HL-60 Cells
The nuclear DNA electrophoresis showed that cells treated with CAM had DNA fragmentation and a low-molecular-weight DNA band between the 200 and 300 base pairs. The fragmentation was due to digestion in the apoptosis process induced by CAM. The molecular results further indicated that CAM treatment can induce cell apoptosis (Fig. 5).
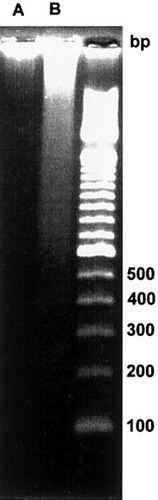
DNA electrophoresis of control (A) and apoptotic (B) cells. The apoptotic cells became so after 4 h of treatment with CAM. The apoptotic cells show typical fragmentation, with a band between 200 and 300 base pairs. Control cells have larger DNA molecules.
FACScan of Apoptotic Cells
To compare the results from the IFC, we also observed control and apoptotic cells on FACScan. The cells treated with CAM showed an 8.9% of the apoptotic cell population as a sub-G1 at the left side of the main cell population and decreased cell numbers at the S phase of the cell cycle (Fig. 6).
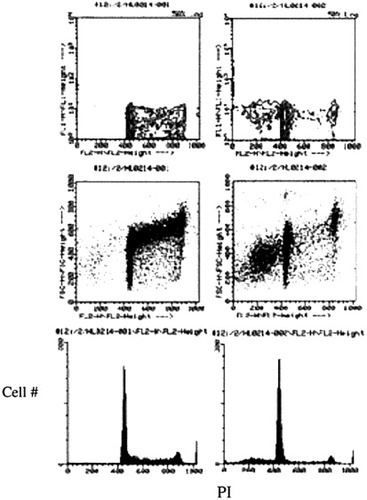
FACScan observation of control (left) and apoptotic cells induced by CAM (right). The CAM-treated cells show increased sub-G1 population (8.9%) due to fragmented and condensed nuclei versus 0.2% of the sub-G1 population in the control.
Apoptotic Cell Observation on the IFC
We successfully observed cell population and morphology simultaneously with the IFC and the newly developed staining method (Fig. 7). We found that the apoptotic cell population increased significantly (9.2% vs. 0.3% of control) after CAM treatment with the IFC. This was identical to the results with FACScan. At the same time, we observed the apoptotic cell morphology in the IFC cell morphology window when we selected any one cell in the apoptotic cell population. The cells, selected at different positions, showed different images including cell size, nuclear shape, and nucleus:cytoplasm ratio. The cells in the apoptotic area were smaller, with fragmented nuclei. These changes are identical to the appearance of cell morphology with the Wright-Giemsa staining method and electron microscopy (Figs. 3, 4).
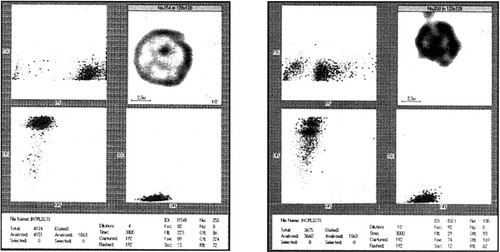
Control and apoptotic cells on the IFC. The control cells show 0.3% of small, low fluorescent cells (equal to sub-G1 on the FACScan). The percentage increased to 9.2 in the CAM-treated samples. These changes are identical to those on the FACScan shown in Figure 6.
DISCUSSION
In the past two decades, research on apoptosis has increased significantly (17) because of its important place in basic, clinical, and biopharmaceutical industrial research. It is understood that apoptosis happens in normal physiologic and many pathologic situations. Apoptotic research is also beginning to shift from fundamental research to clinical application. Many diseases related to cell death actually may be due to the altered apoptosis. New treatments of these diseases are under development based on the understanding of apoptosis. Apoptotic inducers and inhibitors are being designed and used in the treatment of those diseases. Clinically, the correlation of early apoptosis with long-term response in cancer treatment is under investigation. This may become one of, if not the most, widely used methods in the clinical laboratory in the future (18). To conduct all basic research and handle potentially large numbers of clinical samples, fast, automated instruments with reportable multiparameters would be helpful.
Automated methods of apoptotic measurements have been tried with the use of various techniques. Kravtsov and Fabian developed an automated monitoring method to detect apoptosis in culture systems based on apoptotic cell changes in optical properties (19). This method can be used to study the kinetics of cell death in culture.
The research reported in this article was designed to develop a novel method to observe cell population and image or morphology of apoptotic and control cells simultaneously. To do this, we first developed a cell staining method to match the wavelength of the IFC infrared laser beam. After several attempts, we found that HITC and IR-125 can be used for this purpose. The study showed that the cells should be fixed with methanol or ethanol to allow the dyes to penetrate the cell membrane. Fixation also made it possible to stain cell nuclei with PI. HITC and IR-125 are cyanine dyes. They have been used in biologic research (20, 21) but, unfortunately, have not been used in cellular biology. They dissolve well in organic solvent but not in a water-based buffer. We used methanol in the experiment to prepare 0.4% of 10× stock solution. The final concentration of HITC or IR-125 was 0.04% when used to stain cells.
The IFC is a new generation of cytometry based on flow cytometry and imaging technology in accordance with important improvements. The optical beam of the pulse laser is focused 12 μm downstream from the point of focus of the argon–ion laser. This differs from the old prototype (22) because it is necessary to collect the images and side scattered light for the SSC and three colors excited by fluorescent light in a single objective lens. Therefore, the two laser beams have to be focused as closely as possible on one particle. In the IFC, the distance is just 12 μm, which is much closer than the 0.5 mm in the previous prototype.
The IFC was designed mainly for the simultaneous observation of cell populations and images because it is equipped with dual laser beams, one of which is argon and the other is infrared. The argon laser beam has a wavelength of 480 nm, and the infrared beam has a wavelength of 780 nm. The argon laser beam is used mainly in the observation of cell populations by SSC, GFL, RFL, and OFL detection, similar to the traditional flow cytometer. The cells stained with fluorescent dye such as fluorescein isothiocyanate, phycoerythrin, and PI can be grouped according to their fluorescent intensity. The infrared laser beam is another new and critical part of the IFC light source. It is used to observe cell images in different cell populations that are grouped by fluorescent intensity when excited by the argon laser beam or different cell sizes and complexities. The design provides the basic researcher and clinician a tool to observe cell images in different cell populations as they appear in the flow cytometer.
Even though there is some interest in using the IFC in biomedical research and clinical observation, only a few studies have been conducted in these fields. In the case of staining dyes, only auramine O has been used with the IFC (7, 22). Auramine O is a fluorescent dye with an excitation wavelength at 435 nm and emission of 550 nm. This is useful for observing cell populations with the commonly used argon laser beam but useless in an image study because the wavelengths are not in the range of the current IFC image laser beam. We selected three dyes from Exiton, Inc. (Overlook Station, Dayton, OH) and found that HITC showed sharper and brighter nuclear staining, making HITC our first choice in the experiment. The light absorption property of HITC made the cells too dark on the IFC. PBS could not change that action. The cell structure, including nuclei and nucleolus, appeared clearly after washing with 50% methanol. This novel staining method works very well on the IFC cell image observation and makes it easy to observe cell population and morphology at same time on a fast analyzing flow cytometer. There is no doubt that the HITC or IR-125 staining method might be combined with other methods such as fluorescent dye labeled antibodies, terminal transferase-mediated dUTP nick end-labeling, and annexin to provide more cell image information for different cell populations.
With the IFC and the staining method, we observed apoptotic cells. To induce apoptosis, we treated the HL-60 cells with CAM. The CAM-treated cells showed typical apoptotic morphology, nuclear fragmentation, and condensation in light and electron microscopes. DNA electrophoresis showed DNA fragmentation in CAM-treated cells. We examined the behaviors of the apoptotic cells on the IFC and FACScan. Apoptosis induced with CAM provided a valuable model to observe different cell populations and their morphology or image simultaneously. On FACScan and the IFC, cells treated with CAM showed an apoptotic cell population with lower fluorescent intensity after nucleic acid staining. The IFC showed that control cells were larger and had round nuclei and nucleoli, whereas apoptotic cells were smaller and had multifragmented and condensed nuclear pieces when they were stained with HITC. This is identical to the cell morphology with light and electron microscopies.
We have summarized our preliminary apoptotic research with the IFC and the novel staining methods. Both clearly showed the differences between different cell populations. In the corresponding sub-G1 area, the cells showed typical apoptotic morphology similar to what was observed with the Wright-Giemsa staining method. We used HITC and PI staining in the experiment, but other combinations are possible, depending on the research purpose. The newly developed method can be used widely in future biomedical research and clinical tests.
Acknowledgements
The authors acknowledge Mary North for her wonderful technical support in the electron microscope observation and Jill Kutz, Tammy Kutz, and Pam Shearman for their great work in the manuscript preparation and editing.




