FTO-mediated m6A modification of SOCS1 mRNA promotes the progression of diabetic kidney disease
Qiang Sun, Houfa Geng, Meng Zhao, and Yang Li contributed equally to this work, and should be considered as co-first author.
N6-methyladenosine (m6A) is the most prominent and frequent internal messenger RNA (mRNA) modification and plays diverse roles in regulating functions of modified transcripts.1 However, the role of m6A modification in kidney disease remains rarely understood, especially at the onset of diabetic kidney disease (DKD).2 Here, we delineate the biological role of FTO-mediated m6A modification in DKD through imaging mass cytometry (IMC), LC/MS, and RNASeq methods. The results show that the loss of m6A levels by overexpressing FTO recapitulated human DKD by increasing the expression of suppressors of cytokine signalling 1 (SOCS1) protein level to alleviate inflammation response and kidney injury. Thus, FTO maybe a potential therapeutic target for DKD patients.
To quantify the m6A level at cellular and spatial levels, we designed an IMC panel specific to kidney histology and used this to analyse kidney biopsies (Figure 1A and Table S1). IMC integrates IHC using metal isotope-tagged antibodies with laser ablation and mass-spectrometry-based detection to produce high-dimensional images,3 which allow simultaneously quantified the m6A levels and its regulators. We identified 17 676 cells and quantified the levels of m6A, regulators, and spatial characteristics at single-cell level (Figures 1 and S1). We identified five dominant cell clusters of proximal tubules, distal convoluted tubule, glomerulus (Glom) endothelial, macrophage, and stromal cell populations.4 IMC and IHC data demonstrated that the m6A levels were significantly increased in several types of cells, and FTO expression was significantly reduced (Figure 2). The results suggest that FTO plays important roles in m6A dynamics in DKD.
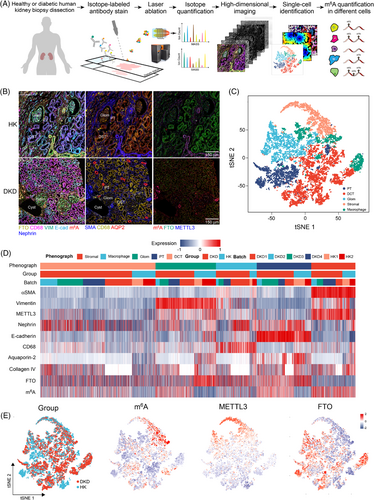
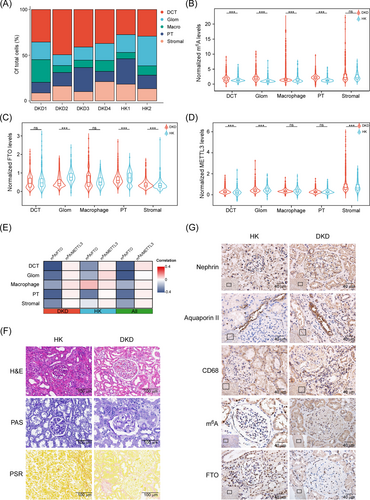
Further analysis revealed that serum m6A levels were significantly upregulated in T2D and DKD patients (Figures S2 and S3a). RNASeq data showed that FTO was significantly downregulated in T2D and DKD patients, whereas other regulators remained unchanged (Figures S3b and S4). Further data also showed that the m6A levels remained significantly upregulated in T2D and DKD patients, and FTO level was negatively correlated with m6A levels (Figure S3c–e). Furthermore, re-analyses of public datasets revealed that FTO was significantly decreased in DKD or uremina patients (Figure S3f–h). To further investigate the role of FTO in DKD pathogenesis, high-concentration glucose (HG) treatment was used to simulate the phenotypes in DKD.5 HG treatment significantly reduced FTO expression, whereas the m6A RNA level was significantly increased (Figure S5a–d). Moreover, other regulators remained unchanged after the HG treatment (Figure S6). FTO overexpression or knock-down significantly reduced or augmented the m6A levels, respectively (Figure S5e–h). HG often triggers glucose-response transcriptional factor ChREBP expression,6 further analysis revealed that the FTO promoter had several ChREBP-binding sites, indicating HG may suppress FTO expression via activating ChREBP (Figure S5i). Together, these data reveal that the m6A modification levels are increased due to FTO downregulation in DKD.
To investigate the molecular mechanism by how dysregulated FTO is involved in DKD. We performed MeRIP-seq in HMC after FTO overexpression, and the results showed that m6A peaks were significantly enriched at the 3′-UTR region (Figure 3A,C) and were characterized by the RAACH motif (Figure 3B). Overall, 25 genes were affected at both RNA expression and m6A levels. Pathway enrichment analyses revealed that the genes affected are involved in inflammation (Figure 3D,E and Tables S6 and S7). More specifically, the m6A level of SOCS1, a key regulator of inflammation, was significantly reduced after FTO overexpression (Figure 3F), which was confirmed by re-analyses of public datasets (Figure S7a). MeRIP-qPCR results showed that SOCS1 m6A level was significantly reduced on FTO overexpression (Figure 3G). Further data revealed that SOCS1 protein level was significantly increased when FTO was overexpressed (Figure 3H). SOCS1 mRNA expression was also positively associated with FTO expression (Figure S7b). Moreover, SOCS1 expression was significantly suppressed after FTO knock-down (Figure S7c). The inhibition of FTO induced by HG treatment could also result in the reduced expression of SOCS1 (Figure S7d). Further results showed that demethylation-inactive mutant FTO (H231A and D233A) had no effects on both m6A and protein level of SOCS1 compared with wild-type FTO (Figures S7e and 3I).Moreover, FTO-RIP-qPCR assays showed the direct binding of FTO on SOCS1 mRNA (Figure 3J). Our findings indicate that FTO can increase the expression of SOCS1 via removing its m6A.
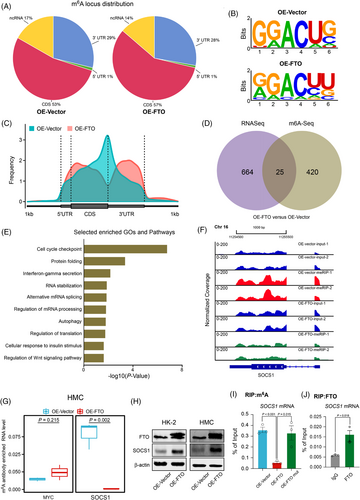
Inflammation plays vital roles in DKD, and SOCS1 is considered an important inflammation regulator.8, 9 Network and GSEA analyses showed that inflammation-related pathways including the JAK-STAT pathway were significantly repressed after FTO overexpression (Figure S8a,b). Moreover, FTO overexpression significantly inhibited inflammation via inhibiting JAK2/STAT3 phosphorylation. In contrast, FTO knock-down and HG aggravated DKD by promoting JAK2/STAT3 phosphorylation (Figure S8c–e). The rescue assay showed that p-JAK2 and p-STAT3 levels were significantly inhibited after SOCS1 knock-down (Figure S8f). Collectively, these results suggest that the JAK-STAT signalling pathway is activated due to the decreased FTO expression.
We generated the db/db mice that were most commonly used T2D/DKD model.10 Intriguingly, injecting fto-overexpressing lentivirus significantly alleviated kidney damage, indicating that fto might be a potential therapeutic target (Figure S9a–g). LC/MS results showed that m6A levels were higher in db/db mice, and m6A levels were decreased after injecting FTO-overexpressing lentivirus (Figures 4A and S9h). qRT-PCR and analyses of public microarray data showed that fto expression was significantly reduced in various mouse models (Figure S9i–m). WB and IHC results showed that both fto and socs1 were significantly reduced in db/db mice, which led to inflammation response via promoting jak2 and stat3 phosphorylation (Figures 4B,C and S10). Surprisingly, fto overexpression significantly increased socs1 expression which alleviated inflammation response as the IHC and WB data showed (Figures 4B,C and S10). The H&E, PAS, SR, and IHC data further indicated that fto overexpression attenuated kidney injuries and fibrosis. Thus, our data suggest that fto overexpression can alleviate kidney injury via suppression of inflammation.
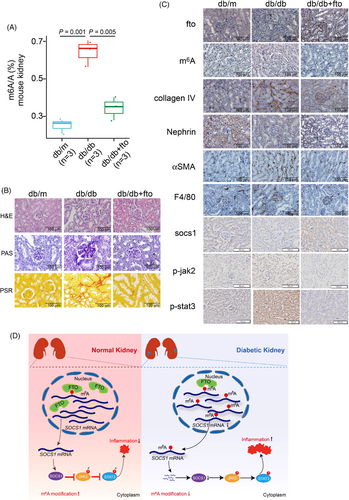
In conclusion, our findings reveal a protective role of FTO during DKD pathogenesis. Mechanistically, the FTO/SOCS1/JAK-STAT axis promotes DKD pathogenesis via promoting inflammation (Figure 4D). Moreover, FTO expression is significantly decreased in DKD, and overexpression of FTO can dramatically alleviate kidney inflammation. Therefore, we suggest that therapeutic targeting of FTO in combination with current therapeutic approaches might be a new avenue for DKD treatment.
DISCLOSURES
No potential conflicts of interest relevant to this article were reported.




