CaMKII, that binds with ligustilide, as a potential drug target of Suxiao jiuxin pill, a traditional Chinese medicine to dilate thoracic aorta
Letter-to-Editor with submission number: CTM2-2022-02-0360
Vasodilation is one of the main means to treat cardiovascular diseases, such as heart failure, cardiogenic shock and severe hypertension.1 Although blocking voltage-gated calcium channels with specific antagonists stands as an optimal therapeutic strategy,2 targeting Ca2+/calmodulin-dependent protein kinase II (CaMKII) may also be a suitable approach, as this enzyme also plays an important role in the regulation of Ca2+-related physiological functions3, 4 and cardiovascular diseases.5, 6 Herein, we reveal a novel mechanism of CaMKII covalent inhibition by one kind of the phthalides present in Suxiao Jiuxin pills (SX), a traditional Chinese medicine frequently used for the treatment of cardiovascular disease.
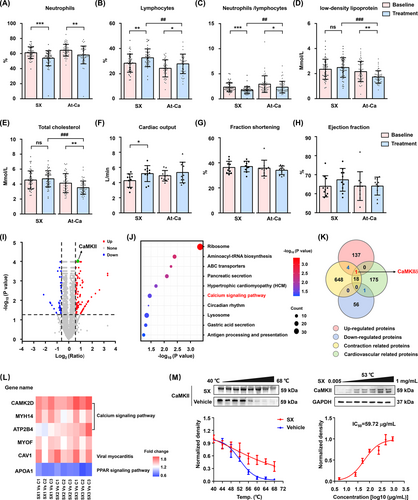
A head-to-head randomized clinical comparison of SX and atorvastatin calcium (At-Ca) in patients with atherosclerosis showed that SX significantly increased cardiac output, while At-Ca mainly lowered lipid overload, indicating that these compounds ameliorate clinical symptoms through different mechanisms (Figure 1A–H, Tables S1 andS2). SX treatment does not affect heart rate and has little effect on myocardial contractility, so it may increase cardiac output by dilating the thoracic aorta (Figures S1, S2). To identify the potential vasodilator targets of SX, a cellular thermal shift assay (CETSA) coupled with iTRAQ-based differential proteomic analysis was performed. The abundance of 199 unique proteins (among which 142 were upregulated and 57 were downregulated) was significantly altered after exposure to SX (Figure 1I). Among them, only one potential target protein was identified, namely, CaMKIIδ which participates in cardiovascular contraction by regulating the calcium signalling pathway (Figure 1J–L). Additionally, CETSA results demonstrated that the SX extract effectively improved the thermal stability of CaMKII in human vascular smooth muscle cells (VSMCs; Figure 1 M).
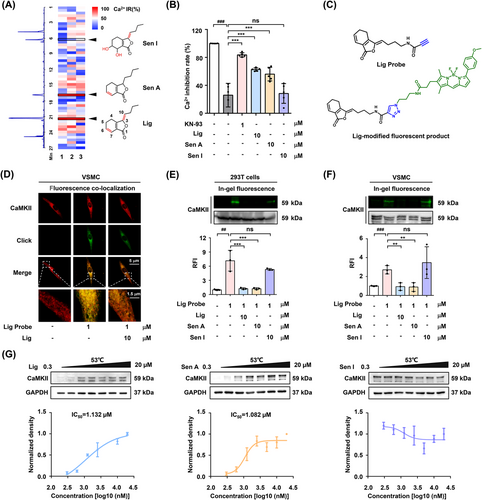
Subsequently, ultra-performance liquid chromatography coupled with CaMKII-mediated calcium antagonist screening was conducted to identify the active pharmaceutical ingredient in SX (Figure 2A). The results revealed that the two major components of SX, ligustilide (Lig) and senkyunolide A (Sen A), exhibited Ca2+-inhibitory activity by acting on CaMKII (Figures 2B, S3). Because Lig was more abundant in SX than Sen A (Figure S4, Table S3), an alkynyl-modified Lig probe and its fluorophore tracer were synthesized and used to verify the interaction between Lig and CaMKII (Figures 2C, S5–S9). VSMCs staining showed that the fluorescence of the Lig-modified tracer partly overlapped with that of the CaMKII antibody, while the overlapping position could be replaced by 10-fold free Lig and Sen A competition (Figures 2D, S10). Moreover, an in-gel imaging assay of 293T cells overexpressing CaMKII and VSMCs indicated that phthalides with double bonds at the C6/C7 might be the key pharmacophores involved in the covalent binding to CaMKII (Figure 2E,F). Furthermore, CETSA results indicated that Lig and Sen A had a similar effect on CaMKII, whereas senkyunolide I (Sen I) had no significant effect (Figure 2G).
In humans, four subtypes of CaMKII share the N-terminal catalytic/autoregulatory domain and a C-terminal association domain, which is necessary for the assembly of the CaMKII holoenzyme complex (Figure 3A).7, 8 To identify the binding site of Lig in CaMKII, the recombinant wild-type human CaMKIIγ protein (residues 1–271) was expressed and purified (Figure S11), then simultaneously incubated with Lig probe and liver tissue lysates. The results of the in-gel imaging assay revealed that only Lig and Sen A covalently bound to CaMKII after liver metabolism (Figures 3B, S12, S13). It is well-established that the C6/C7 double bond of Lig is metabolized into an epoxy group in vivo.9, 10 Protein mass spectrometry identification revealed that the epoxidised metabolite 7-epoxyligustilide (E-Lig) attacks the thiol group of Cys116 on the CaMKII catalytic domain (Figure 3C). Molecular docking indicated that the epoxy group of E-Lig was destroyed, followed by a nucleophilic addition interaction at Cys116 of CaMKIIγ. Meanwhile, the newly formed hydroxyl group established stable hydrogen bond interactions with the Asp-112 and His-115 residues (Figure 3D).
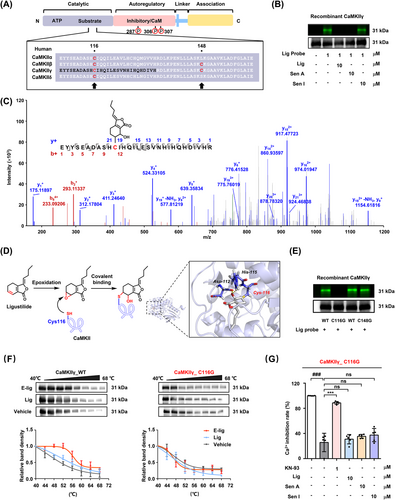
Moreover, CaMKIIγ protein with mutated cysteine residues (C116G and C148G) was utilized to test this hypothesis by in-gel imaging assays. As predicted, E-Lig selectively covalently bound to Cys116 independently of Cys148 (Figure 3E). Thermal stability analysis also supported that only the metabolite of Lig (E-Lig) could covalently bind to CaMKII at Cys116, suggesting this residue may be a druggable site (Figures 3F, S14). Once the Cys116 residue was destroyed, the calcium antagonistic activity of Lig and Sen A in 293T cells overexpressing C116G protein also disappeared (Figures 3G, S15).
To verify the vasodilator effect of Lig in vivo, transthoracic echocardiography was performed to detect the degree of systolic dysfunction in atherosclerotic ApoE−/− mice. Lig treatment effectively reversed the decrease in cardiac output and increased fractional shortening and ejection fraction in a dose-dependent manner (Figures 4A, S16). Co-localization imaging of living tissues revealed that the oral Lig probe mainly targeted CaMKII in the VSMCs of the thoracic aorta (Figure 4B). After oral administration of Lig, the relative fluorescence intensity of phosphorylated CaMKII in the thoracic aorta decreased significantly (Figure 4C). In addition, an experiment with KCl-induced isolated rat thoracic aortic ring indicated that Lig exerts a dramatic vasodilatory effect by inhibiting CaMKII autophosphorylation and its downstream myosin light chain phosphorylation (Figures 4D,E, S17).
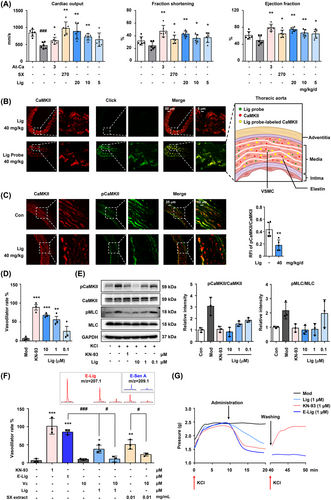
Next, the benefit of the covalent bound between E-Lig and CaMKII on the vasodilator activity was evaluated using an isolated aortic ring test. The vasodilator effect of E-Lig was significantly stronger than that of Lig at the same dose. Remarkably, the effect could be suppressed by a reducing environment (Figure 4F), because the addition of vitamin C (Vc) inhibited the generation of the epoxidised metabolites, both in the Lig and SX extracts. Compared with the CaMKII competitive inhibitor KN-93, the irreversible binding of E-Lig was more enduring for vasodilator activity (Figure 4G).
In conclusion, as a key active component of SX, the epoxidised metabolite of Lig covalently binds to the Cys116 of CaMKII in VSMCs of the thoracic aorta, inhibits the autophosphorylation of CaMKII, and exerts a long-lasting vasodilator effect. Our findings reveal a novel mechanism of inhibiting CaMKII phosphorylation via a nucleophilic addition reaction, providing insights into the development of a potentially promising drug for the treatment of cardiovascular diseases.
ACKNOWLEDGEMENTS
This research was supported by the National Natural Science Foundation of China (Nos. 81673637), and National Key Research and Development Program of China (Nos. 2018YFC1704805).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.




