Altered microRNA expression in severe COVID-19: Potential prognostic and pathophysiological role
Study group members: Lille COVID Research Network (LICORNE)
Lille University Hospital/Centre Hospitalier Universitaire Lille: Comed: LAMBERT Marc; YELNIK Cécile; POKEERBUX Ryad; ANDRE Loïc; BAKHACHE Ed-gar; SCHERPEREEL Arnaud; DESBORDES Jacques; BAUTIN Nathalie; FRY Stéphanie; DUTHOIT Louise; DUHAMEL Nicolas; De GROOTE Pascal; PUISIEUX François; GAXATTE Cédric; BEUSCART Jean-Baptiste; CHARPENTIER Anne; Emergency Department: GOLDSTEIN Patrick; FACON Alain; VANHEEMS FRANCOIS; CUNY Jerome; JOLY Roch; WIEL Eric; CHARBONNIER DINNER Leslie; PEGORARO Vincent; Surgical Critical Care, Department of Anesthesiology and Critical Care: KIPNIS Eric; BOYER-BESSEYRE Marielle; BOULO Marie; BIGNON Anne; BORTOLOTTI Perrine; FAJARDY Marion; Post-operative Intensive Care, Department of Anesthesiology and Critical Care: LEBUFFE Gilles; FACKEURE Remi; CARPENTIER Laurent; ANDRIEU Gregoire; CAPRON Benoit; ONIMUS Jerome; SANDERS Virginie; JEAN-NETEAU Antoine; AMROUN Djihad; Réanimation SALENGRO, Intensive Care Department: BODDAERT Pauline; CAPLAN Morgan; COUSIN Nicolas; DUBURCQ Thibault; DURAND Arthur; EL KALIOUBIE Ahmed; FAVORY Raphael; GIRARDIE Patrick; GOUTAY Julien; HOUARD Marion; JAILLETTE Emmanuelle; JOURDAIN Mercedes; LEDOUX Goeffrey; MATHIEU Daniel; MOREAU Anne-Sophie; NIGEON Olivier; NILES Christopher; NSEIR Saad; ONIMUS Thierry; PARMENTIER Erika; POISSY Julien; PREAU Sébastien; ROBRIQUET Laurent; Dr ROUZE Anahita; Dr SIMONNET Arthur; Dr SIX Sophie; Dr TOUSSAINT Aurélia; Surgical Emergency Department, Department of Anesthesiology and Critical Care: GARRIGUE Delphine; LALLE-MANT Florence; ROHN Aurelien; HENRY Lois; BIJOK Benjamin; STRECKER Guillaume; JOSE-FOWICZ; TAVERNIER Benoit; JOSEFOWICZ Elsa; DEVAUCHELLE Pauline; Cardiothoracic Anesthesia and Intensive Care, Department of Anesthesiology and Critical Care: ROBIN Emmanuel; DECOENE Christophe; AIT OUARAB Slimane; MOUSSA Mouhammed; JOULIN Olivier; LEROY Guillaume; GANTOIS Guillaume; BRANDT Caroline; SADDOUK Noredine; DUPRE Celine; LEROY Xavier; MARIE Raphael; LIU Vincent; FOULON Valentin; MASSIAS Sylvain; RYTTER Nicolas; DEBLAUWE Delphine; PETTIGAND Vincent; MULLER Christophe; DES-BORDES Jacques; DUSSON Catherine; Centre de Biologie Pathologie: BROUSSEAU Thierry; Hémostase—Inserm U1011-EGID: SUSEN Sophie; RAUCH Antoine; LASSALLE Fanny; DUPONT Annabelle; MCU CORSEAUX Delphine; JEANPIERRE Emmanuelle; BAUTERS Anne; TRILLOT Nathalie; Immunologie—Inserm 1286: LABALETTE Myriam; LEFEVRE Guillaume; DEMARET Julie; VARLET Pauline; BOU SALEH Mohamed; Virologie—ULR3610: BOCKET Laurence; ALIDJINOU Enagnon Kazali; PREVOST Brigitte; LAZREK Mouna; TINEZ Claire; MILLIERE Laurine, BOUAROURO Youssef, ENGELMANN Ilka; HOBER Didier; Institut de Microbiologie: SENDID Boualem; Hématologie: NIBOUREL Olivier; Hémostase/Pole BPG: TOURNOY Antoine; Anatomo-pathologie: COPIN Marie-Christine; DUBOIS Romain; GIBIER Jean-Baptiste; GNEMMI Viviane; HUMEZ Sarah; KADRI Malik; LETEURTRE Emmanuelle; PERBET Romain; Centre de Ressources Biologiques/DRI: DEPLANQUE Dominique; Service de maladies infectieuses et tropicales: DOZIER Aurélie; ASSAF Ady; CHOPIN Marie-charlotte; FAURE Emmanuel; VUOTTO Fanny; PANAGET Sophie; FAURE Karine; LEROY Clara; PRASIVORAVONG Julie; BERTHON Céline; GOURSAUD Laure; PROVOT François; LENCI Hélène; BERVAR Jean-François; PREVOTAT Anne
The pathophysiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is beginning to be elucidated but the role of microRNAs (miRNAs), small non-coding RNAs that regulate gene expression, remains incompletely understood. In order to shed light on the role of miRNAs in the pathophysiology of SARS-CoV-2 infection, we have analyzed microRNA (miRNA) expression in nasopharyngeal swabs of coronavirus disease 2019 (COVID-19) patients. We found that miRNA expression was globally reduced in severe COVID-19 and identified several miRNAs of interest to discriminate severe and non-severe COVID-19. Our results suggest that these miRNAs are involved in the pathophysiology of COVID-19 and represent not only promising biomarkers but also possible targets for antiviral or anti-inflammatory treatment strategies.
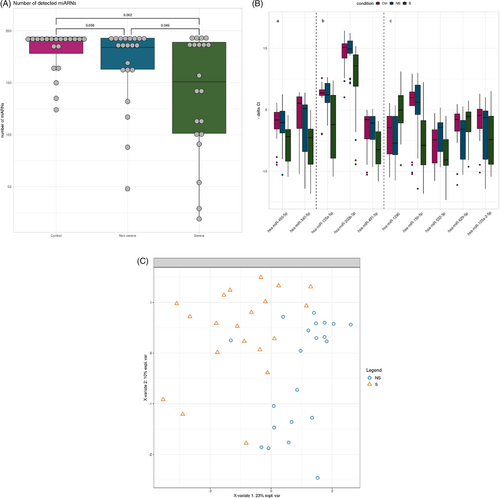
miRNA expression was measured in nasopharyngeal swab specimens from patients with severe, non-severe COVID-19 and controls (Table S1). The number of expressed miRNAs was lower in the severe (median: 151) compared to the other groups (medians non-severe: 184; controls: 190.5) (Figure 1A). Univariate analyses revealed that 14 miRNAs were differentially expressed in severe COVID-19 versus controls (Table 1) and five of these, namely hsa-miR-125a-5p, hsa-miR-200b-3p, hsa-miR-340-5p, hsa-miR-455-5p and hsa-miR-491-5p, were also down-regulated in the severe versus the non-severe COVID-19 groups (Figure 1B, panels a,b; Table 1). A paralleled down-regulation of miRNA expression in severe COVID-19 was reported lately.1 RNA silencing pathways have been identified as antiviral defense mechanisms in plants and insects, and possibly in mammalian cells.2 To escape this antiviral defense, plant and insect viruses possess virus-encoded suppressors of RNA interference, and this may extend to mammalian cells.3 An interesting hypothesis to explain our findings is thus that SARS-CoV-2 targets cellular miRNA biogenesis. In line with this, expression of the RNA interference machinery components Ago2, Dicer and Drosha was significantly down-regulated in COVID-19 patients.4 Furthermore, miRNA depletion enhances proinflammatory cytokine production, including expression of interleukin-6 (IL-6).5 The global repression of miRNA expression in severe COVID-19 that we observed here may thus be causally linked to the hyperinflammatory state found in severe COVID-19.
| miRNA | Log2 fold-change severe COVID-19 versus control | Adjusted p-value | Log2 fold-change severe COVID-19 versus non-severe COVID-19 | Adjusted p-value |
|---|---|---|---|---|
| hsa-miR-125a-5p | −5.170 | .044 | −4.853 | .045 |
| hsa-miR-200b-3p | −3.012 | .044 | −2.752 | .013 |
| hsa-miR-200c-3p | −2.328 | .044 | −1.280 | NS |
| hsa-miR-218-5p | −6.534 | .044 | −5.165 | NS |
| hsa-miR-27a-3p | −2.848 | .044 | −1.790 | NS |
| hsa-miR-30c-5p | −8.041 | .044 | −7.232 | NS |
| hsa-miR-30d-5p | −3.327 | .044 | −1.717 | NS |
| hsa-miR-375 | −1.977 | .044 | −0.859 | NS |
| hsa-miR-378a-3p | −4.365 | <.001 | −1.919 | NS |
| hsa-miR-422a | −5.682 | .002 | 0.067 | NS |
| hsa-miR-455-5p | −2.724 | .044 | −2.260 | .034 |
| hsa-miR-532-5p | −1.898 | .044 | −0.499 | NS |
| hsa-miR-340-5p | −5.601 | .044 | −4.744 | .011 |
| hsa-miR-491-5p | −4.887 | .044 | −4.326 | .031 |
- Abbreviation: NS, not significant.
Multivariate sparse Partial Least Squares-Discriminant Analysis (sPLS-DA) analysis selected eight miRNAs, hsa-miR-125a-5p, hsa-miR-1290, hsa-miR-15b-5p, hsa-miR-491-5p, hsa-miR-532-3p, hsa-miR-200b-3p, hsa-miR-629-5p and hsa-miR-103a-2-5p, that discriminated severe and the non-severe COVID-19 (Figure 1B, panels b,c). Five of these miRNAs were not differentially expressed in univariate analyses (Figure 1B, panel c) but were necessary to build scores with linear discriminant analysis. When using leave-one-out cross validation, 35 out of 41 patients (85.37%) were correctly predicted (Table S2), suggesting that these miRNAs could be used in scores to cluster patients. The coefficients of these scores should be built from a future study that includes more patients and validated in an independent cohort.
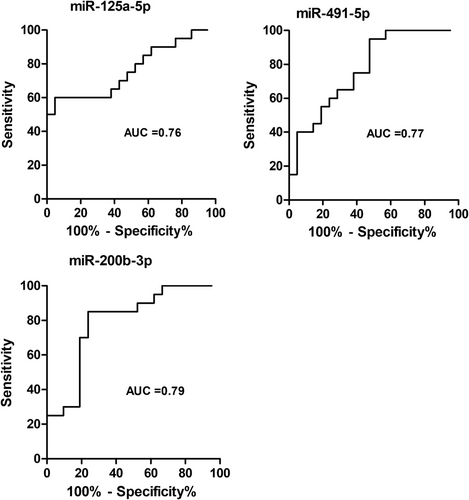
The comparison of results of differential expression analysis and sPLS-DA revealed three miRNA in common, namely hsa-miR-125a-5p, hsa-miR-491-5p and hsa-miR-200b-3p (Figure 1B, panel b). These miRNAs discriminated severe from non-severe cases with areas under the curve ranging from 0.76 to 0.79 (Figure 2). To evaluate their performance as biomarkers to predict disease severity, it would be interesting to determine their expression at different time points, that is, before, during and after resolution of severe COVID-19.
We hypothesized that the 10 miRNAs selected by sPLS-DA and/or differential analysis (Figure 1B) play a role in the pathophysiology of severe COVID-19. Ninety-five validated target genes were retrieved (Table S3). Gene ontology (GO) enrichment analysis revealed enrichment for five, eight and zero GO terms associated with biological process, molecular function and cellular component, respectively (Tables S4 and S5). Enriched biological processes were involved in deoxyribonucleid acid (DNA) damage, ubiquitination and antigen processing and presentation (Table S4). Enriched molecular functions were involved in protein kinase activity, ubiquitination and RNA polymerase II activity (Table S5). Kegg pathway analysis revealed enrichment in pathways that play a role in ubiquitination, viral infections and the immune response (Table 2). Reactome pathway analysis showed enrichment for pathways involved in antigen processing, NFkappaB and other signaling pathways (Table 2).
| Kegg pathway | p-Value | Adjusted p-value (BH*) |
|---|---|---|
| hsa04120_ubiquitin_mediated_proteolysis | .001 | .004 |
| hsa05162_measles | .001 | .004 |
| hsa05169_Epstein-Barr_virus_infection | .001 | .004 |
| hsa05170_human_immunodeficiency_virus_1_infection | .002 | .004 |
| hsa04218_cellular_senescence | .002 | .004 |
| hsa04630_JAK-STAT_signaling_pathway | .003 | .004 |
| hsa05166_human_T-cell_leukemia_virus_1_infection | .008 | .012 |
| hsa05131_shigellosis | .011 | .014 |
| hsa05200_pathways_in_cancer | .032 | .035 |
| Reactome pathway | p-Value | Adjusted p-value (BH*) |
|---|---|---|
| R-HSA-983168_antigen processing: ubiquitination and proteasome degradation | <.001 | <.001 |
| R-HSA-2871837_FCERI mediated NF-kB activation | <.001 | <.001 |
| R-HSA-5607764_CLEC7A (Dectin-1) signaling | <.001 | <.001 |
| R-HSA-9020702_interleukin-1 signaling | <.001 | <.001 |
| R-HSA-202424_downstream TCR signaling | <.001 | <.001 |
- * BH: Benjamini-Hochberg false discovery rate correction
Of the miRNAs found of interest to discriminate between severe and non-severe COVID-19 in our study, some were reported to be implicated in viral and other infections: miR-455-5p was up-regulated in rabies virus infection in vitro, decreased suppressor of cytokine signaling 3 (SOCS3) expression and increased signal transducer and activator of transcription 3 (STAT3) activity, resulting in enhanced viral replication and the production of IL-6.6 Furthermore, this miRNA targeted the C-C motif chemokine receptor 5 (CCR5).7 Of interest, CCR5 is involved in severe COVID-19 and has been proposed as anti-inflammatory treatment target.8 Taken together, this underlines the role of hsa-miR-455-5p in viral infections and the inflammatory response and its potential as target of therapeutic interventions.
hsa-miR-532-3p diminished the levels of ASK1 and downstream phosphorylation/translocation of p38 MAPK during lipopolysaccharide (LPS)/ tumor necrosis factor-α (TNF-α)-induced inflammation in macrophages and reduced the release of various pro-inflammatory cytokines and chemokines, including IL-6 and TNF-alpha.9 IL-6 is a proinflammatory cytokine that has been reported to be involved in the cytokine storm observed in severe COVID-19.10 The down-regulation of anti-inflammatory miRNAs, such as hsa-miR-532-3p, hsa-miR-340-5p and hsa-miR-455-5p, in severe COVID-19 (Table 1) is in line with a hyperinflammatory state in severe COVID-19. Supplementation of these anti-inflammatory miRNAs may represent a novel therapeutic strategy.
Recently, direct and indirect miRNA interactions with other miRNAs have been described (reviewed11). Therefore, it cannot be excluded that the miRNAs identified in this study directly or indirectly influence expression of other miRNAs and thus have a broad impact on miRNA and gene expression.
We next searched for miRNAs that may directly target the SARS-CoV-2 genome among the miRNAs associated with severe COVID-19. We found that hsa-miR-15b-5p was predicted to bind to the SARS-CoV-2 genome at 16 positions (Table S6). Furthermore, hsa-miR-15b-5p was confirmed to interact with SARS-CoV-2 RNA in vitro.12 hsa-miR-15b-5p was down-regulated in severe COVID-19 in our study (Figure 1B, panel c) and in the lungs of hamsters infected with SARS-CoV-2.13 Taken together, this suggests that the down-regulation of hsa-miR-15b-5p may represent a mechanism of SARS-CoV-2 to escape the host antiviral defense.
In conclusion, our analysis of miRNA expression in nasopharyngeal swabs revealed a general reduction of miRNA expression in severe COVID-19 patients. Several miRNAs of interest to discriminate severe and non-severe COVID-19 were identified. Functional analysis of these miRNAs suggested a role in the pathophysiology of the disease. Further characterization of their implication in SARS-CoV-2 infection will enable elucidation of the molecular mechanisms and may reveal potential targets for antiviral or anti-inflammatory treatment of COVID-19.
ACKNOWLEDGEMENTS
The authors thank B. Guerred, S. Menasria and S. Miloudi for excellent technical assistance. They acknowledge the work of technicians and engineers of the Biology and Pathology Center of the Lille University Hospital (CHU Lille) who are involved in the diagnostics of SARS-CoV-2. This research was supported by I-SITE ULNE, the Centre Hospitalier Universitaire de Lille and Université de Lille.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




