Asymmetric bilayer dressings with spatiotemporal sequence loaded with IL-24 and GCDs for the treatment of diabetic wounds
Sijia Li, Jinjin Lu, and Nianqiang Jin contributed equally to this work.
Dear Editor
Diabetic wounds pose a significant global health challenge, affecting hundreds of millions of diabetes patients and imposing substantial financial burdens on healthcare systems.1 Despite conventional treatments, the underlying mechanisms remain poorly understood, resulting in unsatisfactory clinical outcomes. Diabetic wounds exhibit severely impaired fibroblast-to-myofibroblast transition (FMT), a process essential for wound contraction and matrix remodelling. Hyperglycaemia, advanced glycation end products, and chronic inflammation disrupt key signalling pathways regulating FMT, resulting in deficient myofibroblast differentiation and compromised wound healing.2 Recent biomaterial advances in diabetic wound care include hydrogel dressings incorporating growth factors, cytokines, and antimicrobial agents. However, current approaches typically target single mechanisms and fail to simultaneously address impaired cellular function, bacterial infection, and chronic inflammation—the complex interplay characterising diabetic wounds.3 Interleukin-24 (IL-24) exhibits exceptional promise in stimulating cellular proliferation, differentiation, and extracellular matrix production.4 We postulate that IL-24 may facilitate the impaired FMT, thereby enhancing diabetic wound repair. Nevertheless, clinical application encounters significant obstacles, including potential bacterial attraction and challenges in maintaining stable, sustained local delivery at wound sites.5 To address IL-24′s bacterial chemotaxis, we incorporated ginseng-derived carbon quantum dots (GCDs) possessing wide-ranging antimicrobial effects and superior biocompatibility.6 In this study, we discovered significant IL-24 upregulation during diabetic wound healing. Using IL-24 knockout mice, we demonstrated that IL-24 promotes healing by inducing FMT through in vivo and in vitro experiments. Building on these findings, we developed a novel asymmetric dual-layer hydrogel dressing. The tissue side layer delivers IL-24 to enhance FMT, while the outside layer, containing GCDs, provides antibacterial effects through reactive oxygen species (ROS) release. Animal models confirmed this dressing's dual efficacy in promoting wound healing and preventing infection. Our findings establish IL-24 as a promising therapeutic target and demonstrate that this dual-layer hydrogel represents an innovative strategy for diabetic wound management.
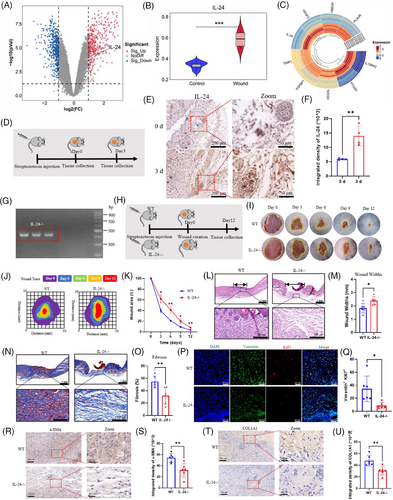
Analysis of gene expression signatures from GEO databases revealed significant IL-24 upregulation in diabetic wounds, demonstrating expression patterns parallel to established wound healing genes (Figure 1A–C). We confirmed these bioinformatic findings through immunohistochemical (IHC) analysis of STZ-induced diabetic C57 mice, which validated elevated IL-24 expression in diabetic wound tissues (Figure 1D–F). To elucidate IL-24′s functional significance, we generated IL-24 knockout (KO) mice and established a diabetic model with dorsal wounds (Figure 1G and H). Planimetric assessment demonstrated that IL-24KO diabetic mice exhibited markedly impaired wound closure (merely 60% by day 6) compared to wild-type diabetic controls (80% closure) (Figure 1I–K). Histological examination through H&E staining corroborated these findings, revealing substantially wider wounds in IL-24 KO diabetic mice (Figure 1L and M). Masson staining identified loosely arranged collagenous architecture and significantly diminished fibrosis ratios in IL-24KO diabetic wounds, indicating compromised fibroblast function during the proliferative healing phase (Figure 1N and O). Notably, myofibroblast populations characterised by α-SMA expression were considerably reduced in IL-24KO diabetic wounds, suggesting dysregulated FMT. Immunofluorescence (IF) analysis revealed attenuated proliferative capacity of myofibroblasts (Ki67+/Vimentin+) in IL-24KO specimens (Figure 1P and Q), while IHC confirmed decreased α-SMA and COL1 expression, indicating impaired extracellular matrix production (Figure 1R–U). Collectively, these findings demonstrate that IL-24 deficiency significantly compromises wound healing progression in type 1 diabetic mice through myofibroblast dysfunction.
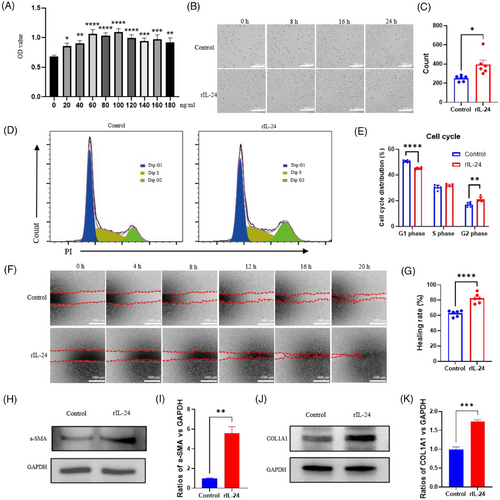
To investigate IL-24′s impact on fibroblasts, we employed L929 cells as an in vitro model. CCK-8 assays identified 100 ng/mL as the optimal concentration for enhancing proliferation (Figure 2A). Previous studies have shown that the in vivo levels of IL-24 range from pg/mL to ng/mL levels. Our 100 ng/mL concentration is well-established in literature, with studies demonstrating optimal IL-24 activity at 50–150 ng/mL in cell culture systems, particularly 75–125 ng/mL for fibroblast applications.7, 8 Time-lapse microscopy confirmed significant cellular expansion following recombinant IL-24 (rIL-24) administration (Figure 2B and C). Flow cytometric analysis revealed elevated cell populations in G1 and G2 phases after rIL-24 treatment, confirming enhanced proliferative capacity (Figure 2D and E). Wound healing assays demonstrated substantially augmented migratory potential in rIL-24-treated fibroblasts (Figure 2F and G). Western blot analysis showed markedly upregulated expression of α-SMA and COL1 in response to rIL-24, indicating enhanced myofibroblast differentiation and collagen production (Figure 2H–K). These findings collectively establish that IL-24 remarkably boosts core fibroblast capabilities crucial for wound recovery, specifically proliferation, migration, and FMT.
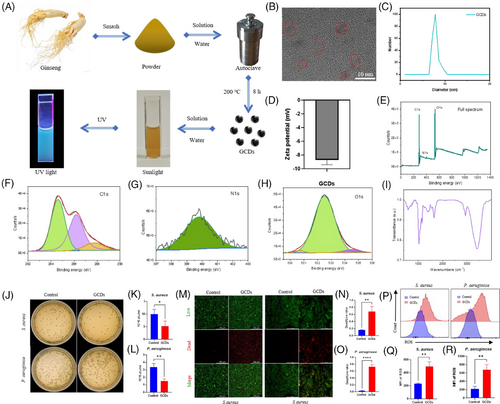
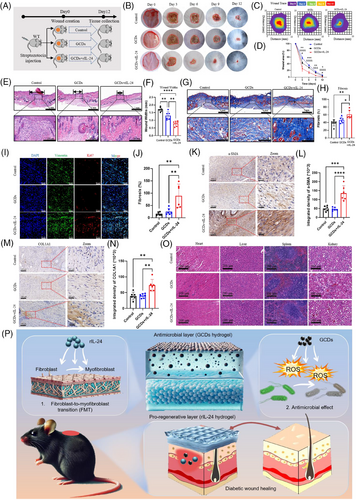
In this study, we identified GCDs from Northeast China ginseng as an effective antibacterial agent. GCDs synthesised through 8-h solvothermal treatment at 200°C demonstrated robust photoluminescence and exceptional fluorescent properties (Figure 3A). HRTEM revealed uniform morphology without aggregation, with approximately 10 nm diameter, confirmed by particle size analysis (Figure 3B and C). The negative zeta potential indicated capacity to attract positively charged bacteria (Figure 3D). XPS and FTIR analyses confirmed C, N, and O as primary constituents, with characteristic vibration peaks at 1750, 2854, and 3400 cm−1 (Figure 3E–I). CFU assays demonstrated significant inhibition of both S. aureus and P. aeruginosa proliferation (Figure 3J–L). Live/Dead staining verified bacterial death, establishing broad-spectrum antimicrobial efficacy (Figure 3M–O). Flow cytometry revealed GCDs substantially enhanced ROS production in both bacterial strains, elucidating their bactericidal mechanism through oxidative stress (Figure 3P–R). The ROS released by GCDs cause negligible damage to normal tissue cells. The differential effects on bacterial versus tissue cells can be attributed to fundamental differences in cellular antioxidant capacity. Mammalian cells possess sophisticated enzymatic defence systems (SOD, catalase, GPx) and compartmentalised organelles for ROS scavenging, while bacterial antioxidant systems are primitive and limited, rendering them more susceptible to oxidative damage. Moreover, GCDs exhibit cytoprotective properties by activating endogenous antioxidant pathways upon cellular uptake, further enhancing the protective effects in tissue cells.9, 10 We employed thermosensitive hydrogel pluronics (HGP) as the delivery vehicle for rIL-24 and GCDs. HGP exhibits excellent fluidity at room temperature while solidifying at body temperature, enabling precise adaptation to diverse wound topographies (Figure S1A and B). This biocompatible carrier achieves sustained rIL-24 release over five days, confirmed through hemolysis tests showing PBS-equivalent safety profiles, with the hydrogel matrix showing controlled biodegradation (Figure S1C–F). To evaluate our composite material's therapeutic efficacy on diabetic wounds, we established mouse models treated with HGP (control), HGP@GCDs, or HGP@rIL-24&GCDs (Figure 4A). H&E staining of major organs confirmed excellent biocompatibility across all formulations, with no detectable abnormalities (Figure 4O). The therapeutic assessment revealed HGP@rIL-24&GCDs significantly accelerated wound closure compared to both control and HGP@GCDs groups (Figure 4B–D). Histological analysis through H&E staining demonstrated significantly reduced wound widths in the HGP@rIL-24&GCDs group (Figure 4E and F). Masson staining exhibited more organised collagen fibre architecture and elevated fibrosis rates following HGP@rIL-24&GCDs treatment (Figure 4G and H). Though GCDs' antibacterial properties modestly enhanced healing, IL-24 emerged as the pivotal therapeutic component. Further investigations revealed HGP@rIL-24&GCDs substantially augmented fibroblast proliferation, myofibroblast differentiation, and collagen synthesis compared to alternative treatments, underscoring IL-24′s crucial role in diabetic wound repair mechanisms (Figure 4I–N). Overall, our research identified IL-24′s critical role in diabetic wound healing through FMT induction, confirmed via knockout studies. We developed an innovative asymmetric dual-layer hydrogel dressing: the tissue-facing layer delivers IL-24 to enhance tissue regeneration, while the external layer incorporates GCDs that release ROS for antimicrobial protection. Animal models validated this dressing's dual functionality in accelerating wound closure and preventing infection. This spatiotemporal approach effectively addresses bacterial vulnerability while maximising IL-24′s regenerative capacity, representing a promising comprehensive strategy for improved diabetic wound management. Although limitations include single diabetic model validation, short-term assessment, and incomplete molecular mechanism characterisation, our thermosensitive hydrogel platform demonstrates exceptional clinical translation potential with practical application advantages and excellent biocompatibility. The dual-functionality approach addresses critical unmet clinical needs, requiring only standardised manufacturing and clinical validation for successful therapeutic implementation. In conclusion, this study provides compelling evidence for IL-24′s therapeutic potential in diabetic wound healing and demonstrates the feasibility of an innovative dual-layer delivery system. While challenges remain for clinical translation, the fundamental scientific advances and therapeutic strategy presented here offer significant promise for addressing the unmet clinical need in diabetic wound management.
AUTHOR CONTRIBUTIONS
Conceptualisation: W.X. Investigation: S.L., J.L., N.J. Formal analysis: N.J., S.H. Writing: S.L., J.L. Funding acquisition: W.X. Supervision: Y.S., J.H.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (82301065); GuangDong Basic and Applied Basic Research Foundation (2022A1515111020); China Postdoctoral Science Foundation (2023M731543); Guangdong Province Natural Science Foundation (General Project)(2025A1515010883); Guangdong Province Medical Research Fund Project (B2025084).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICS STATEMENT
The animal welfare and experimental procedures in this study were approved by the Animal Care and Use Committee of Ruige Biotechnology (No.: 20240410-003).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




