Liver regeneration after injury: Mechanisms, cellular interactions and therapeutic innovations
Qi Liu and Senyan Wang contributed equally to this work.
Abstract
The liver possesses a distinctive capacity for regeneration within the human body. Under normal circumstances, liver cells replicate themselves to maintain liver function. Compensatory replication of healthy hepatocytes is sufficient for the regeneration after acute liver injuries. In the late stage of chronic liver damage, a large number of hepatocytes die and hepatocyte replication is blocked. Liver regeneration has more complex mechanisms, such as the transdifferentiation between cell types or hepatic progenitor cells mediated. Dysregulation of liver regeneration causes severe chronic liver disease. Gaining a more comprehensive understanding of liver regeneration mechanisms would facilitate the advancement of efficient therapeutic approaches. This review provides an overview of the signalling pathways linked to different aspects of liver regeneration in various liver diseases. Moreover, new knowledge on cellular interactions during the regenerative process is also presented. Finally, this paper explores the potential applications of new technologies, such as nanotechnology, stem cell transplantation and organoids, in liver regeneration after injury, offering fresh perspectives on treating liver disease.
1 INTRODUCTION
The liver has a complex structure and performs various functions such as metabolism, detoxification, immunity, haematopoiesis, blood storage, blood volume regulation and coagulation. The liver parenchyma comprises two kinds of epithelium: hepatocytes and cholangiocytes. The non-parenchymal section consists of liver sinusoidal endothelial cells (LSECs), hepatic stellate cells (HSCs), Kupffer cells (KCs), smooth muscle cells, fibroblasts and different immune cells.1 Scientific evidence has demonstrated that the regenerating ability of the adult liver is nearly equivalent to that of a foetal liver.2, 3 When observed in two dimensions, the hepatic lobules create a hexagonal pattern with six inlet areas encircling the central vein (CV).4 The liver lobule is anatomically separated into three distinct regions: Zone 1 located near the junction of the portal vein; Zone 2 situated in the intermediate region; and Zone 3 surrounding the CV.5 Emerging studies indicated that intricately cooperation between hepatocytes and surrounding NPCs are crucial for liver regeneration (Figure 1).
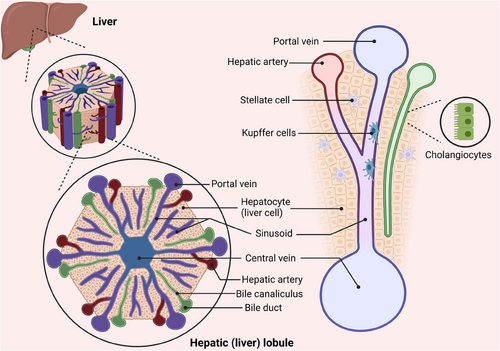
In normal livers, 99% of hepatocytes are in the quiescent (G0) phase, and DNA labelling studies reveal that less than 0.2% of hepatocytes engage in DNA synthesis. Mouse hepatocytes have a life cycle of approximately 200–300 days.3 Under typical physiological circumstances, the replication of hepatocytes is able to sustain the normal functioning of the liver. Upon acute liver injury, the major regeneration mechanism is the compensatory replication of surviving hepatocytes. However, in cases of severe chronic liver disease (CLD), when massive hepatocytes were lost and hepatocyte replication were arrested, the liver will take advantages of other ways, for example, regeneration by liver progenitor cells (LPCs).6, 7 In the past decades, a great deal of study has been done on the basic mechanism of liver regeneration. Hepatocytes are the principal cells responsible for the liver's physiological activities, and their origin is the core of liver regeneration research. The plasticity between cholangiocytes and hepatocytes, as well as their roles as sources of new hepatocytes after injury, have attracted much attention.8 It has been found that in addition to parenchymal cells, non-parenchymal cells (NPCs), such as HSCs and LSECs, also have crucial functions in regulating liver regeneration.9 Moreover, the applications of nanotechnology, stem cell therapy organoids and other emerging technologies provide new research directions and treatment possibilities for liver regeneration.10
In this review, this study provides a concise summary of the most recent research discoveries about liver regeneration in various liver diseases, while also examining the current obstacles faced in this field. The latest interventions associated with liver regeneration and treatment prospects for liver disease were also described. Through these comprehensive studies, this paper hopes to provide new insights into the mechanism of disease-related liver regeneration and provide some ideas for future clinical applications.
2 DIFFERENT RESEARCH MODELS FOR LIVER REGENERATION
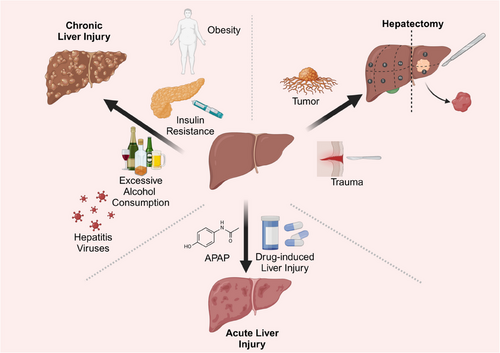
Various models have been used in liver regeneration studies to induce liver injury and subsequent regeneration. Liver regeneration models are categorised into two groups: the post-partial hepatectomy (PHx) models and chemically induced liver injury models.11, 12 These models help researchers understand how the liver responds and regenerates in the context of different types of injury and liver disease (Figure 2).
2.1 The post-PHx models
In PHx models, a surgical procedure is performed to remove a specific section of the liver without causing direct harm to liver cells. This model mainly accomplishes liver regeneration by activating the proliferation of residual liver cells.12, 13 Liver regeneration models of PHx employ the multi-lobular structure of the rodent liver. This involves surgically removing certain lobes without inducing necrosis in the remaining lobes. In rat PHx model, approximately 66% of the liver mass will be excised, whereas in the mouse PHx model, around 50% is taken out.14, 15 The closest human equivalent to this model is PHx that most commonly performed for the resection of primary or secondary liver tumours.16 This model is primarily applied to regenerative capacity study and is able to reliably mimic the regenerative process after human liver resection and is performed in the absence of chemical damage to the liver. In this model, the residual hepatic lobe enlarges to achieve the liver mass prior to PHx within about a week.14 During regeneration, the initial response is hepatocyte hypertrophy, followed by proliferation or hyperplasia of non-epithelial components.17 Following liver regeneration, the lobules and bile ducts enlarge, with the hepatocyte plate width usually increasing from 1 to 1.5 hepatocytes, bordered by hepatic sinuses on each side.6 Multiple studies suggest that in PHx-induced liver regeneration cells such as hepatocytes and cholangiocytes generate new cells of the same kind, characterised by phenotypic fidelity.3, 18 This phenomenon contrasts with the cellular plasticity discussed later. Liver regeneration following PHx also occurs in humans. For instance, liver volume regeneration is most pivotal in the first 2 weeks following living donor transplantation, nearing maximum within 2 months.16, 19 The liver's high regenerative capacity makes the model very valuable for researching fundamental biological processes and regenerative mechanisms. However, the model is more invasive and requires higher surgical skills. Nevertheless, it is incapable of replicating liver damage induced by specific causes, such as viral infection or exposure to chemical toxins.6
2.2 Chemically induced liver injury models
Chemically induced liver injury models, such as the use of diethylnitrosamine, carbon tetrachloride (CCl4) or ethanol, can directly damage liver cells, triggering acute or chronic liver damage.20, 21 These models can more accurately replicate the conditions seen in human liver diseases like hepatitis, liver fibrosis and cirrhosis, making them commonly utilised in drug development and research on treatments for liver disease.
2.2.1 Acute liver injury models
Acute liver injury often occurs when a high dosage of a toxin is given in a single instance. This model is employed to investigate how the liver reacts to acute injury and how it recovers.22, 23 Acute liver injuries are usually caused by short-term, intense factors such as exposure to large doses of chemical toxins. After acute damage to the liver, the remaining liver cells rapidly begin to replicate.24 Liver regeneration mainly relies on the proliferation of relatively healthy residual hepatocytes.11, 25 It can simulate the common clinical situation of drug-induced liver injury. It is highly relevant for studying the immediate response and regenerative processes after liver injury. Acute models may not be able to cover the extracellular matrix (ECM) alterations and inflammatory responses that occur during chronic lesions. The impact on long-term liver function is more difficult to assess.
2.2.2 Chronic liver injury models
Different from acute injury-induced regenerative repair, chronic injury induced by sustained mild or moderate-intensity injury factors, for instance, long-term excess alcohol consumption or chronic viral hepatitis. Chronic liver injury models mimic chronic liver conditions by administering small amounts of chemical triggers like CCl4 or inducing fatty liver through diet for an extended duration (typically spanning weeks to months).26 This simulation replicates the development of long-term liver conditions like liver fibrosis and cirrhosis, making it ideal for researching persistent inflammation, immune reactions and their impact on liver recovery. It requires a longer period of time before pathological changes can be observed. Also, the model is more demanding on animals and more costly to maintain.26, 27 The liver regeneration process is more complex and involves more cell types.28, 29 However, long-period administration of chemoinducers may lead to serious side effects.30 The mechanism of injury of some chemicals may not be fully consistent with the specific mechanism of human diseases.
3 MECHANISMS UNDERLYING LIVER REGENERATION
3.1 Liver regeneration after resection
The liver regeneration procedure after PHx entails the intricate interplay of multiple elements and mechanisms to guarantee the restoration of the liver's functional mass. This process is not only essential for patients undergoing liver resection due to tumours or other diseases but also provides valuable insights into the liver's remarkable regenerative capabilities. Knowledge of the critical factors impacting liver regeneration can help in creating treatment plans to boost recovery and enhance patient results. Many factors influence liver regeneration after resection, such as cytokines, growth factors and bile acids.
3.1.1 Growth factors influencing regeneration after liver resection: hepatocyte growth factor and epidermal growth factor
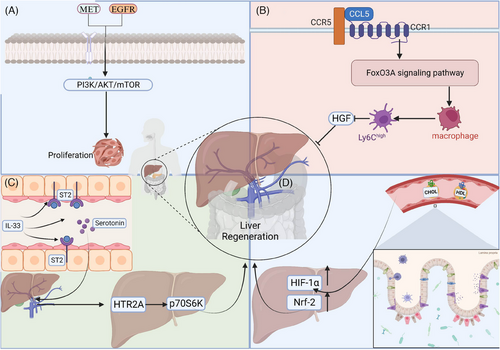
Restoring complete blood flow is crucial for liver regeneration.16 Important molecules that are activated upon the prompt restoration of blood flow are hepatocyte growth factor (HGF) and epidermal growth factor (EGF).31 HGF is secreted by hepatic macrophages and mediates hepatocyte proliferation through its receptor c-Met, while activating downstream effectors such as extracellular signal-regulated kinase (Erk1/2), protein kinase B (AKT) and signal transducer and activator of transcription 3 (STAT3), driving hepatocyte proliferation and survival.32, 33 EGF, through binding its receptor EGFR, initiates the hepatocyte proliferation signalling pathway, promoting hepatocyte proliferation and survival.34 In the PHx model, after blood flow was restored, HGF and EGF were rapidly activated to promote hepatocyte division and proliferation.
The signalling pathways of EGFR and MET are essential for liver regeneration and healthy hepatocyte function. Mice with abnormal MET and EGFR signallings displayed lower liver-to-body ratios, worse hepatic metabolism and higher rates of cell death. This disruption eliminates liver regeneration post-hepatectomy, leading to liver failure and, occasionally, death within 12–14 days after liver resection32 (Figure 3). Pre-treating diet-induced obese mice with meloxicam dramatically enhances the expression of EGFR protein in hepatocytes. Following hepatectomy, meloxicam administration improved liver damage, enhanced hepatocyte division and promoted liver mass regeneration in overweight mice by 70%. Meloxicam treatment post-hepatectomy improved the survival rate of mice by 80%.35 HRX215, a small molecule inhibitor of MKK4 kinase, has been found to effectively promote liver regeneration.36 In a study using a pig liver resection model, HRX215 demonstrated significant efficacy: 85% of the treated subjects, regardless of whether the inhibitor was administered before or after surgery, did not exhibit typical signs of liver failure. The treatment group showed higher survival rates and healthier liver status compared with the control group.36
3.1.2 WNT proteins and signals
The liver regeneration process after hepatic injury relies heavily on the Wnt/β-catenin signalling. Following PHx, the Wnt/β-catenin axis is activated to facilitate quick liver regeneration through stimulating the proliferation and differentiation of hepatocytes. Studies indicate that increased stability and activity of β-catenin directly facilitate hepatocytes enter G1 phase, promoting DNA synthesis and cell division37, 38 Additionally, by controlling downstream effector target genes such as cyclin D1 and c-Myc, Wnt signalling promotes hepatocyte proliferation and tissue repair.39, 40 Post-hepatectomy, Wnt signalling interacts with other signalling pathways like Notch and HGF, forming a complex regulatory network that collectively drives liver regeneration.41, 42
3.1.3 Cytokines
Interleukins like IL-6 and IL33 exert important roles in liver regeneration.31 After hepatic resection and acute liver injury, IL-6 contributes a lot to liver regeneration.43 IL-6, mainly secreted by KCs, activates the Janus Kinase (JAK)–Signal Transducer and Activator of Transcription 3 (STAT3) signalling pathway after binding to its receptor, IL-6R. IL-6–JAK–STAT3 signalling promotes the G1/S transition of hepatocytes via up-regulating cyclinD1expression.43, 44 In addition, IL-6 also enhances hepatocyte survival through the STAT3 signalling pathway.45, 46 STAT3 activation induces the up-regulation of anti-apoptotic genes like Mcl-1 and Bcl-2, thereby enhancing the anti-apoptotic ability of hepatocytes in the injury environment.46, 47 Furthermore, IL-6 plays an important immunoregulation role during liver regeneration. IL-6 regulates the development and function of T cells and B cells, maintains a balanced inflammatory response and protects hepatocytes from excessive inflammation-induced damage.48, 49 Meanwhile, IL-6 promotes the construction of a regenerative environment by regulating the polarisation state of macrophages, which is conducive to the repair and regeneration of hepatocytes.49, 50 Patients having hepatectomy and mice after PHx exhibited increased IL-33 levels. IL-33 promotes regeneration in hepatic resection models, and the deficiency of IL-33 or its receptor ST2 leads to tumour growth inhibition and liver regeneration delay. IL-33 induces intestinal mucosal cells to release more serotonin into the portal bloodstream, activating HTR2A/p70S6K in hepatocytes.51
The chemokine C-C motif chemokine ligand 5 (CCL5) stimulates the proinflammatory development of macrophage through the forkhead box O 3a pathway, which is regulated by CCR1 and CCR5. This process inhibits the generation of HGF and delays regenerative recovery.52 Additional cytokines such as Tumor Necrosis Factor (TNF)-α and transforming growth factor (TGF)-α also have a distinctive influence in controlling the growth and survival of hepatocytes through specific signalling pathways.53
3.1.4 Bile acids
Hepatocytes create bile acids, which are then discharged into the biliary duct and intestines before being absorbed again. Subsequently, bile acids returned to the liver via the enterohepatic circulation. Bile acid levels dramatically increased immediately after PH in rodents and humans.54, 55 Bile acids stimulate the farnesoid X receptor (FXR) and G-protein-coupled bile acid receptor 1 (GPBAR1, also referred to as TGR5), playing essential functions in regulating bile acid balance and preventing liver damage. FXR activation stimulates the expression of genes involved in bile acid synthesis, detoxification and transport. This helps to reduce liver cell damage caused by bile acids and supports the regeneration of the liver. Furthermore, bile acids modulate the activity of various growth and regeneration-related pathways, for instance, the Wnt/β-catenin signalling. Moreover, FXR and TGR5 signalling have been demonstrated not only to promote hepatocytes proliferation but also to protect hepatocytes from apoptosis, thereby facilitating liver regeneration following injury or PHx.56, 57
3.2 Acute liver injury and liver regeneration
Various factors can lead to acute liver injury in clinical settings, including acetaminophen poisoning, alcoholic hepatitis, virus infections like hepatitis A, B and E and autoimmune hepatitis. APAP poisoning is the leading cause of acute liver failure.58 Here, we primarily focus on APAP-induced acute liver injury and regeneration.
3.2.1 Mechanisms of APAP-induced liver injury
APAP is safe at normal doses, but excessive APAP will result in the accumulation of the toxic metabolite N-acetylresorcinol (NAPQI), leading to hepatotoxicity. At normal doses, APAP is metabolised mainly by the hepatic sulphate and glucuronosyltransferase systems.59 Only a small portion is oxidised to form NAPQI via cytochrome P450 enzymes (mainly CYP2E1). When there is too much NAPQI produced in the liver, it surpasses the detoxification ability of glutathione, resulting in the formation of covalent bonds with intracellular lipids and proteins. This leads to cellular damage and death. The buildup of NAPQI causes oxidative stress, DNA damage and disruption of cell signalling pathways, ultimately leading to hepatocyte necrosis.60, 61
Excessive APAP exposure damages mitochondria and results in significant DNA harm, resulting in swift cell death during specific phases of the cell cycle and potentially halting the entire process. Alterations in the activity of lower-level detectors and controllers of DNA damage lead to halting of cell cycle progression at the G1/S checkpoint, postponement of S phase entry and G2 progression. Individuals experiencing sudden liver failure display DNA harm in the liver and abnormalities related to cell division, such as hepatocytes being limited to an abnormal number of chromosomes. However, treating cells with protective cytokines can reverse the APAP-induced cell cycle restriction and restore full circulation.62
3.2.2 Role of P53 in APAP-induced liver injury
P53 has multiple functions in the pathogenesis of acute liver damage induced by APAP poisoning: it prevents further liver damage by maintaining metabolic balance and regulating liver regeneration initiation through proliferative signalling. Experiments revealed that liver regeneration initiation was notably delayed in p53 knockout mice, but once initiated, the cell cycle proceeded much faster than in wild mice due to sustained signalling.63 Moreover, p53 is essential in regulating the response to oxidative stress in APAP-induced liver damage. During an APAP overdose, p53 is stabilised due to the inhibition of its sulfation by PAPSS2, leading to enhanced p53-p21-Nrf2 signalling. This pathway significantly enhances the liver's antioxidative capacity, leading to alleviate liver damage and increased survival rates of mice. The inhibition of p53 sulfation disrupts its interaction with MDM2, preventing p53 ubiquitination and degradation, which further augments its protective effects against APAP-induced oxidative stress. Targeting p53 sulfation as a therapeutic strategy for APAP-induced acute liver failure is emphasised by this mechanism.64
3.2.3 Role of CYP2E1 in APAP-induced liver injury
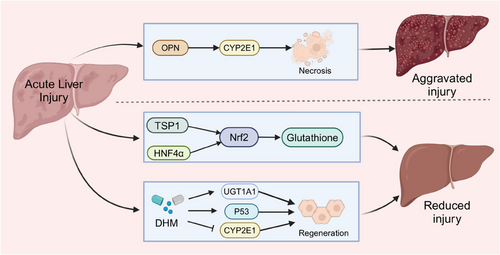
Suppression of cytochrome P450 family 2 subfamily E member 1 (CYP2E1) expression impairs the APAP metabolism. Dihydromyricetin (DHM) mitigates APAP-induced liver injury via regulating proteins that are related to cell death and liver regeneration, including activating UDP-glucuronosyltransferase 1A1 (UGT1A1), promoting p53-associated regeneration and suppressing CYP2E1 expression.65 Furthermore, in a mouse model of hepatotoxicity generated by an overdose of APAP, both serum and liver levels of osteopontin (OPN) showed a considerable rise. OPN protein secretion primarily originates from dying or dead hepatocytes. OPN worsens APAP-induced liver damage by speeding up the breakdown of APAP through increased production of CYP2E1. In addition, while OPN deficiency initially protected against APAP-induced liver damage, it ultimately delayed the healing process by making hepatocytes more susceptible to cell death and hindering liver regeneration66 (Figure 4).
3.2.4 The role of glutathione in APAP-induced liver injury
Multiple researches have proved that adding glutathione is essential for the regeneration and healing of the liver after an overdose of APAP, in which Nrf2 is a critical factor. Thrombospondin-1 (TSP-1), a homotrimeric protein, interacts with proteins like Nrf2 to initiate antioxidant signalling. Additionally, TSP-1 can activate TGF-β1, potentially worsening liver injury. In APAP-treated mice, knocking down the TSP-1 protein decreases TGF-β1 signalling, but results in more liver damage and increased cell death because of lower Nrf2 expression and glutathione activity.58 Hepatocyte nuclear factor 4 alpha (HNF-4α) works with Nrf2 to boost glutathione levels, helping in the healing of liver damage caused by APAP, a process blocked by cMyc67 (Figure 4 and Table 1).
| Relevant factors | Mechanism of action | Impact and consequences | References |
|---|---|---|---|
| p53 | Regulates metabolic balance, liver regeneration initiation, modulates oxidative stress response; stabilised by inhibition of sulfation leading to enhanced signalling and reduced degradation | Delays liver regeneration in knockout mice, enhances antioxidative capacity, improves survival rates, potential therapeutic target for acute liver failure | 63, 64 |
| CYP2E1 | Metabolises APAP; its expression is suppressed by dihydromyricetin, which also activates liver regeneration pathways | Reducing CYP2E1 expression mitigates liver damage, but increased expression through OPN exacerbates damage | 65, 66 |
| OPN (osteopontin) | Secreted by dying hepatocytes, increases CYP2E1 expression, accelerates APAP metabolism | Initially protects against hepatotoxicity, but later sensitises hepatocytes to apoptosis and impairs liver regeneration | 66 |
| Glutathione | Supplementation supports liver regeneration; interacts with proteins like Nrf2 to enhance antioxidant responses | Crucial for recovery post-overdose, modulation by other proteins (TSP-1, HNF-4α) influences liver injury outcomes | 66, 67 |
| ANXA2+ hepatocytes | Facilitates necrotic wound closure, enhances HGF-induced migration and cytoskeletal reorganisation | Critical in liver regeneration; absence reduces migration but not proliferation, interacts with Wnt/β-catenin pathway for liver recovery | 3, 68 |
3.2.5 Discoveries at the single-cell level
The ANXA2+ migratory hepatocyte subpopulation is essential for liver regeneration, primarily by facilitating necrotic wound closure through collective migration.68 The presence of ANXA2+ hepatocyte enhances HGF-induced hepatocyte migration and shows significant cytoskeletal reorganisation during migration.68 HGF signalling promotes cytoskeletal protein reorganisation, thereby enhancing cell migration capability. The absence of ANXA2+ hepatocyte reduces HGF-induced migration but does not affect hepatocyte proliferation. The Wnt/β-catenin axis improves liver function and promotes liver regeneration by controlling the activity of ANXA2+ hepatocytes.3, 68
3.3 CLD and liver regeneration
3.3.1 Mechanisms underlying liver injury in the context of CLD
CLD arises from various sources such as non-alcoholic fatty liver disease (NAFLD), excessive alcohol consumption or viral hepatitis. Alcoholic hepatitis is a condition where the liver becomes inflamed due to long-term and excessive alcohol usage. It is a type of liver disease related to alcohol abuse that can lead to the development of liver fibrosis or cirrhosis.69 Ethanol is first metabolised to acetaldehyde in the liver by enzymes, the main enzymes involved being alcohol dehydrogenase and CYP2E1. Acetaldehyde is further metabolised to acetic acid by the enzyme acetaldehyde dehydrogenase.70, 71 Acetaldehyde is a highly reactive compound that can cause protein and DNA damage, increase oxidative stress and stimulate inflammatory responses.71 Long-term drinking of alcohol can result in fat buildup in liver cells, triggering KCs and other immune cells to release inflammatory substances that may lead to additional liver cell harm and fibrosis.72 Both mechanisms of liver injury involve not only metabolic abnormalities and oxidative stress but also extensive alterations in intracellular signalling pathways.
NAFLD, a common cause of CLD, is mainly linked to metabolic syndrome, obesity, insulin resistance and dyslipidaemia. NAFLD includes a range of liver disorders that go from basic steatosis (accumulation of fat in the liver) to non-alcoholic steatohepatitis (NASH), which may advance to fibrosis, cirrhosis and hepatocellular carcinoma.73 The development of NAFLD is caused by various factors occurring simultaneously, such as insulin resistance causing an increase in free fatty acid flow to the liver, oxidative stress, lipid peroxidation and dysfunction of mitochondria.74
Viral-induced hepatitis, specifically infections caused by hepatitis B virus (HBV) and hepatitis C virus (HCV), are additional major contributors to CLD. HBV and HCV result in persistent liver inflammation, which can ultimately result in the development of fibrosis, cirrhosis and hepatocellular cancer. HBV inserts itself into the host genome and can evade immune surveillance, leading to persistent infection.75 HCV, an RNA virus, induces chronic inflammation through continuous replication and production of viral proteins that trigger immune responses and liver cell injury.76
Regardless of its aetiology, CLD often leads to prolonged hepatocyte loss, resulting in liver fibrosis, cirrhosis and liver tumours.77 Following major hepatic resection or acute liver injury, liver regeneration primarily occurs in relatively healthy remnant hepatocytes, a process often referred to as physiological regeneration. In these situations, mature hepatocytes restore liver function through proliferation. Although LPCs play a lesser role in these cases, they may be activated and participate in regeneration when hepatocytes have limited proliferative capacity or are excessively injured.
In CLD, liver regeneration is more complicated. LPCs-mediated regeneration is particularly pronounced in these contexts and is usually accompanied by the transdifferentiation of hepatocytes and cholangiocytes, a phenomenon referred to as pathological regeneration. Overall, the main feature of regeneration in chronically injured livers is the coexistence of hepatocyte self-replication and regeneration mediated by LPCs or other cells. The dominance of particular mechanisms relies on the extent of hepatocyte proliferative capacity impairment and the seriousness of the injury. During the initial phases of many chronic liver conditions, hepatocyte self-replication may still predominate, but LPCs or other cell-mediated regeneration may become more important as the disease progresses and the injury worsens.
3.3.2 Liver regeneration originated from biliary epithelial cells
Recent studies have elucidated the complexity of liver regeneration in chronic disease.78-80 For instance, research has highlighted the role of transitional LPCs (TLPCs) in situations when hepatocyte-mediated regeneration is compromised. TLPCs originate from biliary epithelial cells (BECs) and possess the ability to differentiate into hepatocytes.80 A dual genetic lineage tracing method was used in the research to mark TLPCs and monitor their differentiation trajectory. TLPCs were discovered to have the ability to differentiate into hepatocytes or revert back to a BECs fate, demonstrating their bipotency. This plasticity is essential for liver regeneration under conditions where hepatocyte proliferation is limited. Mechanistically, the study showed that Notch signalling is pivotal in maintaining the BECs identity and preventing their transition to TLPCs.80 On the other hand, the Wnt/β-catenin pathway encourages TLPCs to develop into fully functional liver cells. The inhibition of Notch signalling in BECs was shown to enhance the activation of TLPCs, increasing their conversion to hepatocytes.80
Researchers used snRNA-seq and advanced 3D imaging on liver biopsies from patients at different MASLD stages to uncover notable alterations in liver structure and cell activity. They discovered that hepatocytes lose their zonation and that biliary tree undergoes considerable reorganisation.78 They discovered that hepatocytes lose their zonation and that biliary tree undergoes considerable reorganisation. Crucially, the conversion of hepatocytes to cholangiocytes happens without the involvement of adult stem cells or developmental progenitors.78 Cholangiocyte organoids demonstrated the importance of the PI3K–AKT–mTOR pathway in functional validations, connecting it to insulin signalling.78, 79 This pathway, along with others such as Wnt/β-catenin and TGFβ signalling, orchestrates the cellular plasticity necessary for liver regeneration in the face of chronic injury.
3.3.3 Liver regeneration originated from LPCs
LPCs are oval-shaped liver resident stem cells located in Hering's duct at the junction of liver parenchymal cells and the confluence area, resembling biliary BECs in morphology and volume. LPCs show the presence of stem cell indicators like EPCAM, Sca-1, CD133, CD24, A6, Trop2 and Lgr5, in addition to cholangiocyte markers SOX, CK19 and hepatocyte markers HNF-4α, CK8. LPCs are bipotent stem/progenitor cells that are capable to differentiate into both hepatocytes and cholangiocytes.24 LPCs also have other sources, for example, in some circumstances, mature hepatocytes or cholangiocytes can act as parthenogenetic stem cells, transforming into each other to restore normal liver architecture architecture.3
Hepatocytes, which are fully developed liver cells, can undergo a process called ductal metaplasia under chronic injury, where they transform into hepatic progenitor cells. This transformation is reversible and allows the cells to avoid further damage. Once the injury stops, these hepatic progenitor cells can then differentiate back into fully functional hepatocytes.81 CD24+LCN2+ LPCs are mainly derived from non-substantial cells of the liver, such as BECs and existing LPCs. Recently, it has also been demonstrated that hepatic progenitors of non-hepatocyte origin make a very limited contribution to the regeneration of the mouse liver.82
In vitro, the transformation of hepatocytes and hepatic progenitor cells can be induced by replicating a human environment that is conducive to liver regeneration. The in vivo environment supporting liver regeneration was mimicked using specific small molecules and growth factors, such as HGF in combination with Y-27632, A-83-01 and CHIR99021, which resulted in the in vitro conversion of mature hepatocytes into proliferative-expandable hepatic progenitor cells.81, 83, 84 Meanwhile, co-culturing human LPCs (HepLPCs) with human umbilical vein endothelial cells (HUVECs) effectively mimicked the in vivo microenvironment of the human liver, and the glial cell-derived neurotrophic factor secreted by the HUVECs facilitated the transformation of HepLPCs into mature hepatocytes through activation of the Met signalling pathway.85
3.3.4 Signalling pathways for the LPCs differentiation
Extensive studies have been conducted on signalling pathways that regulate LPCs differentiation, including Wnt/β-catenin, Notch, HGF/c-Met and Hippo/YAP signalling. Wnt/β-catenin signalling is associated with LPCs differentiation into hepatocytes. In rats treated with 2-ethoxytoluamide and major hepatic resection, β-catenin activity significantly increased during LPCs proliferation. Conversely, LPCs numbers decreased substantially without β-catenin, indicating its key role in LPCs activation and proliferation.34
It has been demonstrated that the phagocytosis of hepatocyte debris by macrophage cells induces Wnt3a expression and activation of classic Wnt/β-catenin signalling in neighbouring LPCs, leading to differentiation into hepatocytes.86 Research have shown that interfering with or excessively stimulating Wnt/β-catenin signalling can hinder the formation of the biliary tract within the liver of zebrafish. Suppression of the Wnt/β-catenin pathway leads to decreased Notch function in BECs. Inhibiting Wnt/β-catenin signalling in hepatocytes reduces Notch activity in BECs. The levels of JAG1B and JAG2B Notch ligand genes decrease in liver cells when Wnt/β-catenin signalling is blocked, and rise in liver cells when Wnt/β-catenin signalling is heightened. These findings indicate that the liver's Notch activity is controlled by Wnt/β-catenin signalling. Crucially, the restoration of Notch activity can effectively fix the damage to the biliary system produced by the suppression of Wnt/β-catenin87 (Figure 5).
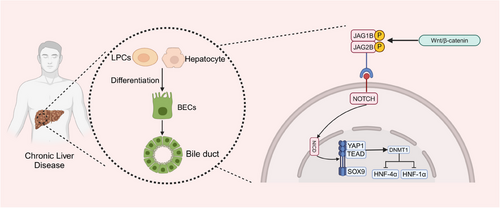
The Notch pathway is a highly conserved controller of cell growth and the upkeep of stem/progenitor cells in different tissues. It has been demonstrated to have significant functions in the differentiation of bile ducts. Alagille syndrome, caused by congenital Notch deficiency, results in biliary defects and subsequent cholestasis. For instance, Alagille syndrome, which is caused by a congenital deficiency in Notch signalling, leads to biliary defects and cholestasis.88 During biliary regeneration, myofibroblasts express Jagged1, promoting Notch signalling in LPCs and facilitating their differentiation into cholangiocytes.89 EpCAM controls the transformation of LPCs into hepatocytes through the activation of the Notch1 pathway. Genealogy tracking experiment has revealed that Notch–RBPJ signalling is vital in bile duct regeneration and in vitro differentiation of LPCs to cholangiocytes, underscoring the central role of Notch signalling in LPCs differentiation.90, 91 Furthermore, studies have indicated that the NOTCH–YAP1/TEAD–DNMT1 axis is critical for the transformation of hepatocytes into BECs. The Notch intracellular domain (NICD) independently regulates SOX9 and YAP1. Inhibiting either YAP1 or TEAD can hinder this transdifferentiation. DNMT1, a downstream effector of the YAP1–TEAD complex, directs hepatocyte transformation to BECs by repressing hepatocyte-specific genes such as HNF-4α, HNF-1α and CCAAT/enhancer-binding proteins α/β. DNMT1 deletion prevents the NOTCH/YAP1-mediated hepatocyte transdifferentiation to BECs and the resulting cholangiocarcinogenesis. In vivo, time-lapse imaging has shown that the conversion from hepatocytes to BECs occurs independently of a proliferative intermediate stage92 (Figure 5 and Table 2).
| Signalling pathway | Role in LPC differentiation | Key interactions and effects | References |
|---|---|---|---|
| Wnt/β-catenin | Promotes LPC differentiation into hepatocytes | Activation by macrophages phagocytosing hepatocyte debris, leading to hepatocyte differentiation. Inhibition or over-activation can disrupt biliary tract development. | 34, 86, 87 |
| Notch | Regulates proliferation and maintenance of stem/progenitor cells | Involved in bile duct differentiation. Reactivated in BECs during regeneration, aiding in cholangiocyte differentiation from LPCs. EpCAM activates Notch1 to drive differentiation. | 88-92 |
| HGF/c-Met | Essential for LPC transformation into hepatocytes | Activates downstream pathways (e.g., Erk1/2, AKT, STAT3) promoting differentiation. C-Met deficiency impairs this ability. | 53, 93 |
| Hippo/YAP | May regulate LPC differentiation | Ectopic expression of YAP reprograms mature hepatocytes into LPCs, capable of differentiating into hepatocytes and cholangiocytes. | 94 |
HGF is crucial for inducing LPCs transformation into hepatocytes. HGF triggers the activation of the c-Met receptor, leading to the activation of downstream effectors such as Erk1/2, AKT and STAT3, ultimately promoting the differentiation of LPCs. The absence of the c-Met receptor impairs LPCs proliferation and migration.53 HGF/c-Met-mediated Akt and STAT3 activation is necessary for differentiation from LPCs to hepatocytes. c-Met deficiency leads to the loss of LPCs' ability to differentiate into hepatocytes.93
The Hippo–YAP pathway is essential for controlling the differentiation of LPCs. YAP ectopically expressed in mature hepatocytes transforms them into LPCs.94, 95 Recent research has offered a more profound understanding of the intricate systems that underlie this pathway.94 For instance, a single-cell RNA sequencing study revealed significant heterogeneity in YAP expression among cholangiocytes in a 3,5-diethoxycarbonyl-1,4-dihydrocollidine injury model. This heterogeneity indicates that different subsets of cholangiocytes may respond differently to injury and regeneration cues. Additionally, bile acids have been demonstrated to control YAP activation, a crucial factor in preserving biliary reactions in the event of liver damage and recovery.94 Further studies have also emphasised the interaction between the Hippo–YAP pathway and various signalling pathways like Wnt and Notch, influencing the behaviour of LPCs and liver regeneration.96 The results highlight the significance of the Hippo–YAP pathway in liver function and its promise as a target for treating liver disorders.96, 97
3.4 Cellular interactions in liver injury and regeneration
Liver regeneration primarily focuses on the proliferation of hepatocytes, which requires the coordinated efforts of several NPCs, including LSECs, HSCs and KCs. Interactions among hepatocytes and NPCs form a complex regulatory network, essential for restoring liver mass and function. Understanding these complex intercellular communications is vital for a deeper insight into the regeneration process.
3.4.1 Interaction of KCs with hepatocytes
As previously mentioned, liver regeneration involves interactions between various cell signalling molecules. KCs located between the endothelium of hepatic sinusoids and hepatocytes account for about 80% of the body's total macrophages.98 Upon liver injury, increased blood flow activates the synthesis and release of HGF and EGF. Afterwards, KCs are activated by these stimuli to generate and release TNF-α. TNF-α functions in an autocrine manner regulated by NF-κB. NF-κB also triggers the release of IL-6. IL-6 binds to its receptors on the cell surface of hepatocytes, activating the JAK–STAT3 pathway. This enhances the progression of hepatocytes from G1 to S phase by increasing the expression of cyclin D1, thereby promoting cell cycle initiation and replication of hepatocytes.98, 99 Research has demonstrated that KCs can impede the process of liver regeneration by releasing substances such as TGF-β.99 The communications between KCs and hepatocytes are crucial in liver regeneration.
3.4.2 Interaction of LSECs with hepatocytes
LSECs located in the hepatic sinusoids, are the first to contact hepatic blood flow and are also the earliest cells to be damaged in liver diseases. LSECs, unique endothelial cells with abundant window pore structures and no intact basement membrane constitute approximately 70% of the liver's NPCs.100 LSECs secrete diverse cytokines that are crucial for inducing hepatocyte regeneration and maintaining the quiescence of HSCs.101 Sources of LSECs in liver regeneration encompass bone marrow-derived sinusoidal endothelial precursor cells (BM-SPCs), mature LSECs and resident liver SPCs. Precise synchronisation between LSECs and hepatocytes is essential for liver regeneration. LSECs coordinate the release of cytokines and growth factors to promote hepatocyte proliferation, while hepatocytes also control the proliferation of LSECs. During PHx, there was an increase in hepatic vascular endothelial growth factor expression, leading to the recruitment of HGF-rich BM-SPCs through the stromal cell-derived factor-1 (SDF-1)/CXCR7 axis.102 Nitric oxide (NO) secreted by LSECs sensitises hepatocytes to HGF, promoting liver regeneration.103 During APAP-induced acute liver injury, LSECs secrete Wnt proteins and enhance HGF expression in the repair phase. This LSECs originated Wnt signal promotes β-catenin activation in hepatocytes, thus up-regulating target genes like cyclin D1 and driving hepatocyte proliferation.104 In CCl4-induced acute liver injury, LSECs-mediated activation of c-Kit pathway facilitates liver repair via Wnt2-dependent manner.105 In studies on NASH, LSECs secrete cytokines and extensively interact with cholangiocytes, HSCs and other NPCs. In LSECs of NASH livers, expression of genes related with lipid metabolism was up-regulated and those for vascular homeostasis were down-regulated, resulting in the destruction of capillaries in hepatic sinusoids. LSECs also express DLL4 and TGF-β1, which interact with corresponding receptors on monocytes, leading to their differentiation into hepatic macrophages.105, 106
3.4.3 Interaction of HSCs with hepatocytes
HGF is essential for regulating the multiplication of hepatocytes, making it a key growth factor. Hepatocytes receive HGF through both endocrine and paracrine manner.107 HSCs that have been activated, located in the Disse space between hepatic sinusoidal endothelial and hepatic epithelial cells, are the main producers of HGF.108 During liver injury and other pathological conditions, HGF binds directly to c-MET, a hepatocyte surface receptor, initiating hepatocyte proliferation. HGF's binding to c-MET activates downstream pathways such as the MAPK cascade, PI3K–Akt–STAT3 axis and NF-κB pathway.109 During early liver regeneration stages, HSCs produce norepinephrine. This reduces the inhibitory impact of TGF-β on mitosis and boosts HGF and EGF secretion.110 During the terminal phase of regeneration, HSCs separate excess growth factors by reorganising the ECM, causing hepatocytes to stop dividing.111 Concurrently, the activation and differentiation of LPCs, as previously mentioned, occur amidst multiple cellular interactions. Damaged hepatocytes directly activate HSCs. Meanwhile, macrophages, monocytes, T-lymphocytes and hepatic sinusoidal endothelial cells modulate HSCs activity by secreting factors like IL-6, TNF-α, HGF and TGF-α/β.112, 113 These intercellular interactions and signals uncover complex regenerative mechanisms in the liver after injury (Table 3).
| Cell or cellular component | Involved cytokines/pathways | Relationship to other cells | References |
|---|---|---|---|
| KCs | TNF-α, IL-6, TGF-β, JAK–STAT3 pathway | Promotes hepatocyte proliferation via cell cycle proteins; also inhibits hepatocyte regeneration | 98, 99 |
| LSECs | HGF, NO, DLL4, TGF-β1, Wnt/β-catenin pathway, c-kit pathway | Enhances hepatocyte response to HGF; promotes hepatocyte replication via cell cycle proteins; activates stem cells; promotes monocyte differentiation | 100-106 |
| HSCs | HGF, EGF, c-MET, MAPK pathway, PI3K–Akt–STAT3 pathway, NF-kb pathway | Generation of HGF to promote hepatocyte proliferation; remodelling of ECM to terminate regeneration | 107-113 |
| ECM | Integrin signalling, MAPK pathway, PI3K-Akt-STAT3 pathway, Wnt/β-catenin pathway | Affects hepatocyte proliferation and migration; regulates immune cell behaviour and macrophage phenotype via cytokines and chemotactic factors | 114-119 |
3.4.4 Interaction of ECM with hepatocytes
Upon liver injury, the composition of the ECM undergoes significant changes, and these changes have a profound effect on hepatocyte behaviour and liver regenerative capacity. For example, increased deposition of collagen and elastin enhances ECM stiffness, which in turn affects hepatocyte proliferation and migration.114 The ECM is not only a passive structural framework but also regulates intracellular signalling pathways by binding to cell-surface integrins.115 It has been shown that increased ECM stiffness can activate multiple intracellular signals, including MAPK, PI3K/Akt and Wnt/β-catenin pathways.116, 117 Changes in the ECM during liver injury affect not only hepatocytes but also the behaviour of immune cells such as KCs. These cells can secrete cytokines and chemotactic factors that further regulate the composition and function of the ECM, forming a complex network of interactions. For example, TGF-β produced by macrophages can stimulate HSCs to produce more collagen and exacerbate hepatic fibrosis, and altering the structure of the ECM can also influence macrophage phenotype and function.27, 118, 119
4 INTERVENTIONS AND THERAPEUTIC PERSPECTIVES IN LIVER REGENERATION
Over the past few decades, there has been a rise in groundbreaking technologies focused on liver regeneration, presenting new treatment possibilities for a range of liver conditions. These technologies range from bioartificial systems that mimic liver functions, to advanced methods like normothermic machine perfusion (NMP) that preserve liver integrity before transplantation. Alongside, nanomaterials are being explored for their therapeutic potential, while stem cell technologies pave the way for cell replacement therapies. Additionally, organoids provide a novel approach for in vivo liver regeneration, showcasing how these advancements collectively push the boundaries of medical science towards effective liver treatment and recovery.
4.1 Normothermic machine perfusion
NMP maintains liver activity prior to transplantation by supplying oxygen and nutrient-rich blood through a circulatory system that mimics that of the human body, keeping the liver functioning at body temperature.120 NMP helps to reduce the duration of oxygen deprivation, optimise metabolic status and reduce the risk of liver damage after transplantation.120, 121 Studies have shown that NMP significantly improves the success rate of transplantation of borderline quality livers, especially from cardiac arrest donors.122, 123 NMP can be utilised for drug testing and metabolic research to evaluate the possibility of liver function and injury recovery, as well as to expand the pool of livers for transplantation.123
4.2 Nanomaterials
Nanomaterials, with their unique properties such as small size, surface and quantum effects, are considered potent tools for treating liver diseases. They were used for drug delivery, imaging and as scaffolds for cell growth.124 Nanoparticles made of cerium oxide, which are effective at removing reactive oxygen species, greatly speed up the process of liver regeneration in rats that have experienced liver injury from post PHx and APAP overdose. These nanoparticles induce hepatocyte proliferation, reduce stress markers, advance cell cycle progression and activate NF-κB transcription factors.125 Tetrahedral framework nucleic acids (TFNAs), known for their antioxidant and anti-inflammatory properties, activate various proliferative and prosurvival pathways. TFNAs stimulate hepatocyte proliferation and liver repair in mouse models of 70% PHx, APAP overdose and CCl4 injury by triggering the Notch, Wnt and P53 pathways.126 Additionally, a study highlighted the potential of using magnetic nanoparticles to attract stem cells to migrate to damaged liver areas.127 These findings indicate that nanomaterials and nanostructures hold substantial potential for enhancing liver regeneration and offering new therapeutic options for liver disease.
4.3 Stem cell-related technologies
Stem cell-related technologies, particularly pluripotent and adult stem cells, present a promising approach for cell replacement and liver regeneration. Stem cells, capable of differentiating into hepatocytes, also play vital roles in liver preservation through anti-inflammatory, immunosuppressive, angiogenic and anti-apoptotic mechanisms. Additionally, research indicates that paracrine factors from stem cells promote angiogenesis, decrease inflammation and inhibit hepatocyte apoptosis.127 Various stem cells, especially mesenchymal stem cells (MSCs), are used in liver therapy. MSCs are derived from adult or perinatal tissues like bone marrow, placenta, umbilical cord, liver and adipose tissue. In a CCl4 liver injury model, stem cells from umbilical cord blood successfully differentiate into hepatocyte-like cells, thus ameliorate liver damage levels and fibrosis degree and also restoring liver functions evidenced by decreased aminotransferase and increased albumin levels.128 Transplanting induced MSCs derived from embryonic stem cells in rats post PHx reduce bilirubin levels and promote liver regeneration in vivo.
Recent studies have highlighted the role of MSC-derived or bone marrow MSC-derived extracellular vesicles (EVs) as novel therapeutic agents.129, 130 These EVs can package and deliver bioactive molecules such as RNA, DNA, proteins and lipids that modulate inflammation, reduce fibrosis and promote tissue regeneration.131, 132 This cell-free treatment may offer a safer alternative to direct stem cell transplantation, reducing the risk of cell integration and proliferation.132
4.4 Organoids
Organoids, a recently developed biological model, have become instrumental in liver regeneration research. They are three-dimensional, organ-like structures generated from stem or organ-specific cells cultured in vitro.133 Organoids, when grafted in vivo, can connect to host blood vessels through vascularisation, maturing more effectively than in vitro.134 Researchers created liver-like organs (LOs) with hiPSC-derived endoderm, endothelial cells and mesenchymal stromal cells from a single donor and transplanted the LOs into mice with acute liver failure. The transplant mice showed improved liver function and increased survival rates in a short time.135 Primary cells isolated from rat livers can expand chronically into organoids or LPCs under the induction of Wnt agonists, TGF-β inhibitors or the injury-induced inflammatory cytokine TNF-α. Under these conditions, rat hepatocyte derived organoids were able to expand in vitro for approximately 2−3 months and express bile duct markers as well as LPCs markers. Notably, these expanded rat hepatocyte-like organs recapitulate the proliferation pattern of hepatocytes after PHx in terms of gene expression pattern.136, 137 A research study documented the creation of functional hepatobiliary organoids from hepatocytes.138 This group of organoids consists of a system of bile ducts encircled by fully developed hepatocytes and effectively preserves hepatic traits and functionality both in laboratory settings and following transplantation into living organisms.138 Inhibiting Tead4 or increasing Ddit3 expression can reverse hepatocyte fate decisions during in vitro and in vivo transplantation, uncovering the key transcription factors that control hepatobiliary cell fate determination in the liver.138 In addition, it has been reported that adult hepatocytes can be induced into LPCs in vitro and expanded in long-term culture under certain culture conditions. However, when these expanded LPCs were further cultured into organoids, they redifferentiated into mature hepatocytes and showed significant improvement in liver function as well as a significant reduction in hepatic progenitor cell characteristics.84, 139
Currently, it is possible to construct proliferating human hepatocytes (ProliHHs) by dedifferentiating primary human hepatocytes to a dual phenotype state without genetic manipulation.140, 141 ProliHHs have hepatocyte and progenitor cell characteristics, that can expand at least 10,000-fold, and, in a 3D organoid system able to redifferentiate to a mature state close to primary human hepatocytes.140 In ProliHH-like organs, hepatic gene expression and corresponding liver function are significantly improved.141 Intraperitoneal transplantation of encapsulated ProliHHs liver organoids (eLO) was performed on animals with liver failure.142, 143 In a mouse model of liver failure after 80% hepatectomy, eLO significantly improved survival and provided normal liver function, resulting in amelioration of hyperammonaemia and hypoglycaemia.144 Furthermore, eLO therapy safeguarded the gut barrier, decreased endotoxin levels and suppressed inflammation, ultimately facilitating the regeneration of the liver. The effectiveness of the treatment was also validated in a mouse model with excessive APAP-induced liver failure.144 And eLO caused no adverse effects in mice and was consistently non-tumourigenic.144 Treatment of two mouse models of liver failure demonstrated the efficacy of eLO intraperitoneal transplantation and confirmed the safety of eLO, providing a solid foundation for ongoing organoid clinical studies. It is promising that in the future, doctors may use patient-specific cells to culture organoids, enabling personalised treatment regimens. This approach mitigates sample availability and ethical concerns associated with allogeneic transplantation and lessens the need for immunosuppressive drugs in patients.
Cholangiocyte organoids are miniature biostructures generated in vitro using stem cell technology that mimic the physiological function and structure of real bile ducts.145 These organoids can be applied for bile duct repair after liver transplantation. Cholangiocyte organoids can be established by inducing differentiation of human pluripotent stem cells, such as induced pluripotent stem cells (iPSCs) or adult stem cells, under specific culture conditions.146, 147 Studies demonstrated the possibility of using human cholangiocyte organoids to repair damaged bile ducts.145, 147 The researchers proved that human iPSCs-derived cholangiocyte organoids not only possess normal bile duct function in vitro, but also promote repair and regeneration of the damaged bile ducts after transplanted into the damaged bile duct region.146, 147
4.5 Bioartificial liver
The bioartificial liver (BAL) system is a device that uses living cells to simulate liver functions, providing temporary life support to patients with acute or chronic liver failure.148 Recent research developments have shown that these systems can not only filter toxins from the blood but also secrete liver-specific proteins such as albumin and transferrin, potentially facilitating liver regeneration.149, 150
Presently, BAL systems predominantly employ two types of hepatic cells: primary hepatocytes and stem cell-derived hepatocytes.151 Primary hepatocytes are typically sourced from human or animal livers, but the difficult availability and variable quality limit their application. Consequently, increasing researchers are utilising human pluripotent stem cells derived hepatocytes, which express hepatic marker genes and possess liver cell functions, such as protein synthesis, urea production and glycogen storage.152
Animal model studies have demonstrated that the BAL system significantly improved survival rates and liver functions in acute liver failure.153 More inspiringly, the BAL system has shown promising results in clinical trials, that treating acute liver failure patients with the BAL system safely improved their immunoglobulin levels and significantly enhanced short-term survival rates.154 With technological advancements and the accumulation of clinical data, the BAL system is poised to become an effective supplement or alternative to liver transplantation, particularly where there is a shortage of liver donors.
The advancements in liver regeneration technologies signify a monumental shift in therapy for liver diseases. From the precision of nanomaterials in drug delivery and liver repair to the sophisticated developments in organoid and BAL systems, these technologies collectively enhance our capability to treat and manage liver repair more effectively. As research progresses, these innovations are promise to improve liver therapies and broaden the scope of recoverable liver conditions, ultimately improving patients’ outcomes and life quality.
5 DISCUSSION
In the current study, we have thoroughly explored various aspects of liver regeneration, particularly the liver's self-repair capabilities following acute and chronic liver injury. While several signalling pathways, including the Wnt/β-catenin, Notch and Hippo/YAP pathways, have been linked to liver regeneration, the precise functions and interplay of these pathways in various liver injury types are still not well known. The Wnt/β-catenin pathway, for example, is essential for liver formation and regeneration, but more research is needed to understand how it regulates specific liver diseases and how it affects LPC behaviour. Furthermore, although the Notch pathway is important in controlling the plasticity between cholangiocytes and hepatocytes, little is known about how it contributes to the development of liver fibrosis and whether it has any potential therapeutic uses.
Further research is necessary to fully understand the liver's capacity for regeneration in individuals suffering from chronic liver disorders such cirrhosis and liver fibrosis. In these cases, the liver's ability to regenerate is severely restricted, and exploring how to activate and enhance this capability without causing liver cancer is a crucial direction for future research. Meanwhile, although the study of LPCs has provided a new perspective on liver regeneration, their specific roles and potential in different types of liver injuries still need further exploration. For example, the mechanisms behind the activation and differentiation of LPCs following chronic liver injury and how these mechanisms are influenced by the surrounding microenvironment remain current research hotspots.
Approaches for enhancing liver regrowth with stem cells and organoid technology have displayed potential, yet substantial obstacles need to be addressed for successful implementation in a clinical setting. For instance, ensuring that liver cells regenerated through these methods have adequate functionality and long-term stability, as well as avoiding potential immune rejection or malignant transformation, are key issues that future research must address.
Further studies should concentrate on uncovering the molecular processes involved in liver regeneration and how this information can assist in treating liver disorders. The increasing incidence of CLDs and other liver conditions urgently demands new treatment strategies. We anticipate progress in treating liver diseases with ongoing technological advancements and research developments.
AUTHOR CONTRIBUTIONS
Xiaofang Zhao and Hongyang Wang planned the project. Jing Xu, Wenjuan Wei and Hao Song collected relevant information. Yao Chen, Jing Fu and Senyan Wang determined the structure of the article. All authors analysed the data. Qi Liu wrote the paper. All authors discussed the results and commented on the manuscript.
ACKNOWLEDGEMENTS
This study was supported by the following grants: Natural Science Foundation of Henan Province, Project No. 242300420070; the Open Project of Key Laboratory of the Ministry of Education, Project No. 2022-MEKLLC-ZD-002; National Key Research and Development Program of China, Project No. 2022YFC3400902; National Natural Science Foundation of China, Project No. 82073411. All six figures in this manuscript were created with BioRender.com.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of financial interests.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data are available from the authors upon request.




