Longitudinal circulating tumour DNA dynamics predict failure patterns and efficacy of consolidation immunotherapy after chemoradiotherapy in locally advanced non-small-cell lung cancer
Dear Editor,
Responses to consolidation immune checkpoint inhibitor (ICI) in locally advanced non-small-cell lung cancer (LA-NSCLC) are heterogeneous, and current decision-making procedures have little accuracy.1 This prospective cohort study provided the first evidence for dynamic circulating tumour DNA (ctDNA) predicting failure patterns in LA-NSCLC patients receiving chemoradiotherapy (CRT), allowing the early identification of the potentially curable population with radical CRT and different therapeutic benefits from consolidation ICI.
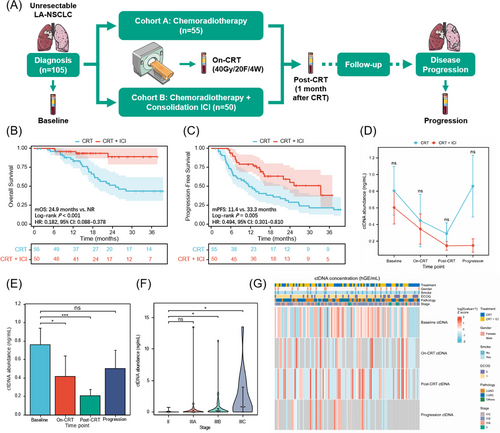
Exploring effective biomarkers to guide personalized consolidation immunotherapy, avoid overtreatment, and reduce the potential risk of immune-related toxicities is of clinical importance.1-3 In this prospective multicenter trial (NCT04014465), 105 patients with unresectable LA-NSCLC were assigned to CRT or CRT plus consolidation ICI cohorts, with balanced baseline characteristics (Figure 1A and Table S1). As expected, patients undergoing consolidation ICI had significantly improved overall and progression-free survival (PFS) (Figure 1B,C). All patients have collected blood samples at baseline, on-CRT (radiotherapy reached 40 Gy/4 weeks), post-CRT (1 month after CRT), and progressive timepoints, subjected to 486-gene next-generation sequencing to analyze longitudinal ctDNA. Detailed information about ctDNA assay techniques and study procedures is attached as the Supporting Information. No significant difference in ctDNA abundance across cohorts at any time point (Figure 1D). Notably, quantitative ctDNA could reflect tumour burden,4 since ctDNA levels significantly decreased with effective CRT but increased at disease progression, and baseline ctDNA positively correlated with the clinical stage (Figure 1E–G).
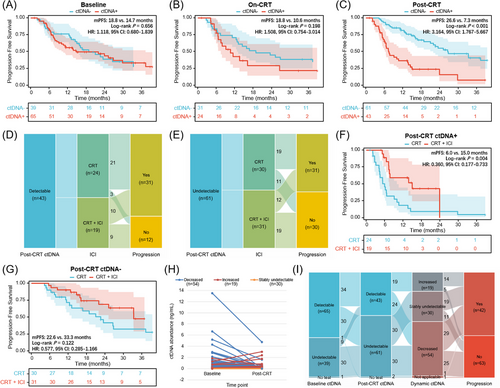
We further explored the prognostic value of ctDNA at longitudinal landmark timepoints. Post-CRT detectable ctDNA, rather than baseline or on-CRT ctDNA, was associated with significantly worse survival (Figure 2A–C). In patients with detectable ctDNA post-CRT, 75% of non-progression patients received consolidation ICI, while 63.3% of non-progression patients used ICI in the undetectable population (Figure 2D,E). Survival analyses were confirmed in patients with detectable ctDNA post-CRT, those receiving consolidation ICI had significantly longer PFS than those without ICI, yet no significant difference in patients with ctDNA clearance (Figure 2F,G). According to post-CRT ctDNA minus baseline ctDNA levels, the dynamic change pattern of longitudinal ctDNA included decreased (n = 54), stably undetectable (n = 30), and increased (n = 19; Figure 2H). Strikingly, the majority of patients with increased ctDNA (73.7%) developed disease progression (Figure 2I).
Next, we investigated the predictive effect of longitudinal ctDNA on first failure patterns, which were categorized as local-regional, distant, or both, as presented in Figure 3A. The primary failure pattern for patients with decreased ctDNA in the CRT cohort was distant metastasis (51.9%), and consolidation ICI significantly reduced the incidence of distant metastasis (18.5%; p = .019). However, in the stably undetectable ctDNA group, the incidence of local-regional failure (21.4% vs. 37.5%) and distant metastasis (28.6% vs. 31.3%) was both similar between patients with and without consolidation ICI (p = .592). In the increased ctDNA group, simultaneously local-regional and distant failure was the most predominant failure pattern (36.4%) for patients with CRT, and consolidation ICI brought a tendency (p = .583) of a reduced proportion of local-regional (18.2% vs. 12.5%), distant (27.3% vs. 25.0%), and simultaneously local-regional and distant (36.4% vs. 12.5%) failure.
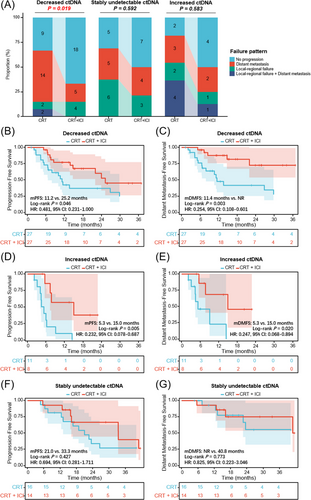
For patients with decreased ctDNA post-CRT, although consolidation ICI brought significant PFS benefit (p = .046; Figure 3B), a more obvious distant metastasis-free survival (DMFS) benefit was observed (p = .003; Figure 3C). In the increased ctDNA population (Figure 3D,E), patients with consolidation ICI had significantly improved PFS (p = .005) and DMFS (p = .020). In contrast, in the stably undetectable ctDNA group (Figure 3F,G), no significant difference in PFS (p = .427) or DMFS (p = .773) between patients with and without ICI. These data suggest that dynamic ctDNA could identify different failure patterns and therapeutic responses to ICI: 1) Decreased ctDNA dynamics predict a higher risk of distant metastasis but more DMFS benefit from ICI, presumably because effective radiotherapy could cause localized tumour cell death and accordingly decreased release of tumour-derived products into the peripheral blood, whereas it is difficult for local radiotherapy to completely eradicate disseminated ctDNA pre-existed in the circulation.5 Consolidation ICI as a potent systemic treatment would effectively reduce the risk of overt metastases and micrometastases, thereby bringing DMFS benefit to this patient subset; 2) Increased ctDNA, reflecting resistance to definitive CRT, is associated with a higher risk of distant and local-regional failure, and subsequent ICI improves DMFS as well as PFS. In the clinical setting, for patients with solitary lesions of suspected metastasis,6 dynamic ctDNA testings may contribute to differential diagnosis between primary and metastatic diseases, as increased ctDNA indicates the hematogenous spread of tumour cells in the peripheral circulation7 3) Stably undetectable ctDNA predicts excellent outcomes irrespective of consolidation ICI, suggesting the potentially curable population with CRT.2
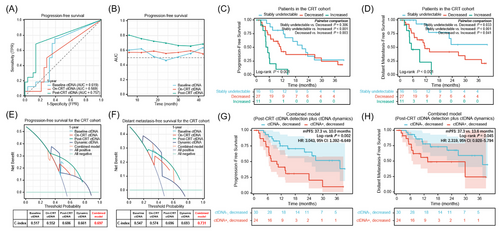
Cox regression analysis demonstrated consolidation ICI, post-CRT ctDNA, and dynamic ctDNA changes were significant variables in predicting PFS (Table S2 and Figure S1). Lastly, we compared the prediction effects and clinical decision-making benefits of single-time ctDNA detection with longitudinal ctDNA dynamics. Time-dependent receiver operating characteristic curves indicated that post-CRT ctDNA was the optimal predictor of PFS (Figure 4A,B). In terms of dynamic ctDNA assessments, as shown in Figure 4C,D, patients with increased ctDNA had the significantly worst PFS and DMFS, and patients with stably undetectable ctDNA had the longest survival. Furthermore, decision curve analysis suggested the combined model integrating qualitative post-CRT ctDNA detection with quantitative ctDNA dynamics had superior usefulness in predicting PFS and DMFS, compared to individual features (Figure 4E,F). Based on this combinatorial model, patients with decreased ctDNA were further divided into two groups, and patients with post-CRT detectable/decreased ctDNA had significantly worse PFS and DMFS than those with undetectable/decreased ctDNA (Figure 4G-H), in both CRT and CRT + consolidation ICI cohorts (Figure S2).
In conclusion, we determined that the multiparameter assay integrating qualitative with quantitative ctDNA metrics could improve patient risk stratification, consistent with previous findings.8-10 Moreover, we innovatively discovered dynamic ctDNA could predict failure patterns, potential curability with CRT, and different responses to consolidation ICI, thereby serving as a robust prognostic and predictive model with improved usefulness to facilitate ctDNA-guided treatment personalization. Our findings warrant independent validation in a larger-scale clinical cohort. The ongoing randomized, phase II trial (InTRist, NCT05888402) will further validate the predictive value of longitudinal ctDNA dynamics and provide high-quality evidence for translational application of ctDNA in LA-NSCLC.
AUTHOR CONTRIBUTIONS
Yu Wang and Tao Zhang performed data analysis and interpretation, investigation, as well as manuscript drafting. Yin Yang, Jianyang Wang, Canjun Li, Xin Xu, Yuqi Wu, Ying Jiang and Jinghao Duan assisted with data acquisition and curation, project administration, and independent validation. Luhua Wang and Nan Bi supervised this study, provided resources, acquired funding, and critically revised the manuscript. All authors contributed to the study conceptualization and approved the final version.
ACKNOWLEDGEMENTS
We thank the patients, caregivers, and their families for participating in this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
This work was founded by the National Natural Sciences Foundation Key Program (No. 82173348), CAMS Innovation Fund for Medical Sciences (No. 2021-1-I2M-1-012), and the Special Research Fund for Central Universities, Peking Union Medical College (No. 3332023133).
ETHICS STATEMENT
This study was approved by the institutional review board of the Chinese Academy of Medical Sciences (No. 19/098-1883). All patients provided written informed consent.




