Tumorigenicity and prediction of clinical prognosis of patient-derived gastric cancer organoids
Ting Wang, Wanlu Song and Qingyu Meng contributed equally to this work.
In this study, we established a biobank of gastric tumour organoids (GTOs), which retained primary tumour characteristics. The response of GTOs to drugs was correlated with individual patient's long-term postoperative prognosis, suggesting that GTOs are a good model for drug prediction for gastric cancer (GC) treatment.
Varying response to chemotherapy restricts GC treatment efficacy and clinical prognosis.1 GC-derived organoids are a good model for drug tests as they preserve primary tumour features.2-5 However, the correlation between patients' long-term clinical prognosis and drug sensitivity is unclear. The distinction between GTOs and gastric normal organoids (GNOs) is vague. To address these questions, we established GTOs and corresponding GNOs from 17 GC patients: seven intestinal-type, eight diffuse-type and two mixed-type (Table S1).6 Two types of GTO morphology were observed: one displaying a glandular, round pattern with an obvious lumen, similar to intestinal-type tumours; the other exhibiting a solid morphology with the nucleus shifting to one side and resembling diffuse-type tumours (Figure 1A,B). GTOs derived from mixed-type GC tissues displayed both types of morphology (Figure 1C). All GNOs derived from normal tissues displayed glandular structures, mimicking normal gastric glands (Figure 1D).
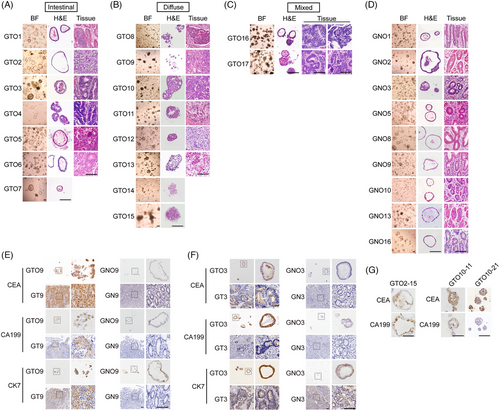
To assess GTOs’ tumour features, gastric tumour markers carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and cytokeratin 7 (CK7) were examined. CEA and CA19-9 expression were detected in GTO2, GTO3, GTO9, GTO13, GTO2-15, GTO10-11 and GTO10-21 and their corresponding GTs, but not in GNOs and normal tissues (Figure 1E–G and S1A-C). CEA and CA19-9 expression were found in 13 GTOs and their corresponding GTs (11/13 and 12/13, respectively) (Table S2). Therefore, GTOs preserved tumour marker expression of tumours, even in long-term cultures.
Whole exome sequencing revealed analogous tumour mutational burden in GTOs (Figure S2A and Table S3). Furthermore, gene mutations were correlated with specific clinical features (Figure S2B-D and Table S4). Single nucleotide variants for different passages of GTO9 and GTO10 exhibited significant similarity to primary tumours (Figure S3A,B). While gene mutations in GT9 were retained in long-term passages of GTO9, some GTOs acquired additional mutations during prolonged culture (GTO12-22 and GTO17-22) (Figure 2A), possibly due to growth competition among different subclones.7 The base substitutions exhibited notable similarities in most matched organoids and GTs (Figure 2B). A substantial proportion of mutations were common mutations (Figure 2C,D). Mutational signatures were also similar between GTOs and GTs (Figure 2E, S3C and Table S6). These findings suggest that GTOs effectively preserve mutation characteristics of primary tissues.
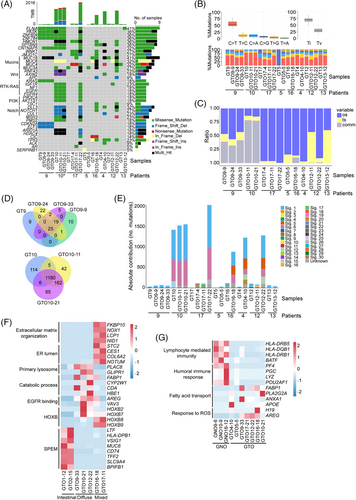
Gene expression profiling revealed a robust correlation between GTOs and GTs (Figure S4A and Table S7). Principal Components Analysis showed a close proximity within GTO subtypes (Figure S4B,C). Compared to GT, GTOs had high expression in genes linked to xenobiotic stimuli and low in the ones associated with immune response and fibroblasts (Figure S4D). Distinct gene expression profiles were also observed in three types of organoids (Figure 2F). Compared to GNOs, immune-related genes (lymphocyte-mediated immunity and humoral immune response) were downregulated in GTOs (Figure 2G).
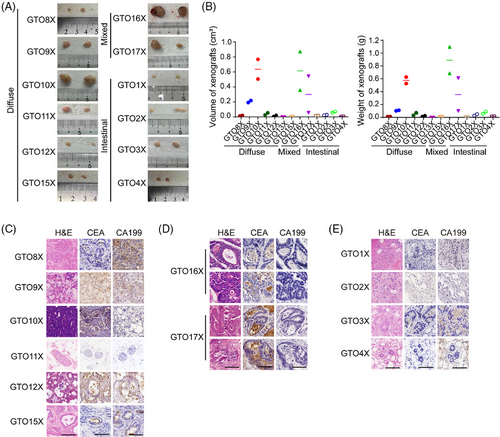
To evaluate GTO tumorigenicity, we conducted GTO xenograft (GTOX) experiments. Most diffuse-type and mixed-type GTOs displayed strong tumorigenicity, and the GTOXs exhibited similar structures to the original GTs and were CEA+ (Figure 3). However, some GTOs had low or no tumorigenicity, and the GTOXs displayed connective tissue-like neoplasms (Figure 3). GNOs only formed cysts (Figure S5A,B). Variations in GTO tumorigenicity are possibly linked to WNT mutations, which requires further exploration (Figure S5C).
To simulate the therapeutic effects of drugs, GTOs were treated with various drugs. The drug resistance of GTOs was correlated with late stages of GC but had no significant associations with gender, age, or tumour classification (Figure 4A and Table S8). GTO10 was more sensitive to oxaliplatin than to 5‑fluorouracil (5‑FU)/oxaliplatin (Figure 4A and Table S9), consistent with MSI-H status8 (Table S5) and good outcome of oxaliplatin monotherapy in this patient (Figure S6A). Conversely, patients 4, 6 and 9 treated with 5-FU/oxaliplatin had a poor outcome and exhibited resistance in GTOs. A significant correlation was also observed between progression-free survival time and cell viability in GTOs (Figure S6B). However, some GTO responses did not align with clinical outcomes, possibly due to peritoneal metastasis hindering drug delivery (patient 3). GTO4, which highly expressed TOP1MT, was sensitive to irinotecan,9 indicating potential as a second-line treatment (Figure 4B and S6C, D). GTO10, with PIK3CA mutation, showed resistance to erlotinib and gefitinib (Figure 4A,C), and GTO9 with KRAS mutation exhibited sensitivity to gefitinib.10 These data underscore GTOs’ role in assessing drug response and optimizing treatment strategies, along with gene expression and mutations. Furthermore, most drugs showed consistent responses in GTO2, GTO12 and GTO17 with over 10 passages apart (Figure 4D).
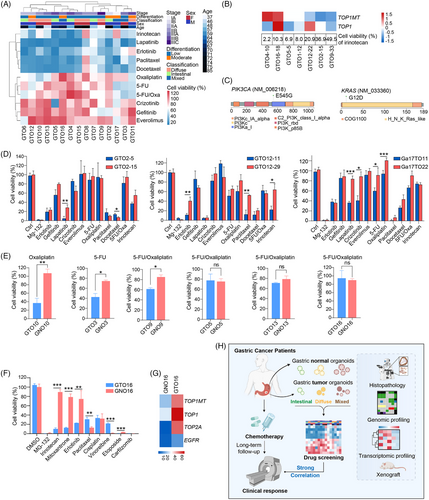
We then evaluated drug side effects using GNOs. Oxaliplatin exhibited low toxicity in GNO10 although displaying high efficacy to induce cell death in GTO10 (Figure 4E), consistent with patient 10's positive response to oxaliplatin. Similar results were observed in organoids of patients 3 and 9. Irinotecan, mitoxantrone and erlotinib demonstrated significant efficacy in GTO16 with minimal cytotoxicity in GNO16 (Figure 4F, S6E and Table S8). Likewise, mitoxantrone and erlotinib showed a similar pattern, consistent with gene expression (Figure 4F,G and S6E). Therefore, GNOs can be used for drug toxicity assessment.
In summary, our findings demonstrated that GTOs can faithfully recapture tumour features of original tumours and preserve genetic alterations after long-term culture. We found distinct expression profiles among GTO subtypes, reflecting clinicopathological features of GC subtypes. Finally, we observed consistency between clinical prognosis and drug sensitivity in GTOs, indicating the potential use of GTOs for personalized medicine.
AUTHOR CONTRIBUTIONS
Ting Wang and Ye-Guang Chen conceived the study, analyzed the data and wrote the manuscript; Ting Wang and Chuanqing Qu performed the experiments; Wanlu Song performed bioinformatics analysis; Baoqing Jia, Qingyu Meng and Shaohua Guo provided specimens; Yalong Wang and Ronghui Tan revised the manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (31988101) and the Natural Science Foundation of Jiangxi Province (20224ACB209001) to Ye-Guang Chen.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The approval number is 2019−346 from the PLA General Hospital Ethics Committee.
Open Research
DATA AVAILABILITY STATEMENT
The genomic data are in https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE235912.




