The combination of computed tomography-derived muscle mass and muscle density and relationship with clinicopathological characteristics and survival in patients undergoing potentially curative surgery for colorectal cancer
Abstract
Background
Sarcopenia has been defined as a loss of muscle mass and function. CT-derived muscle measurements, skeletal muscle index (SMI) and density (SMD), taken together may provide an objective measure of sarcopenia. The aim of the present study was to examine the relationship between CT-derived sarcopenia (low SMI and SMD), clinicopathological characteristics, systemic inflammation and survival in patients undergoing surgery for colorectal cancer.
Methods
Consecutive patients who underwent resections for colorectal cancer (TNM I–III) at our institution, between April 2008 and 2018, were identified from a prospectively maintained database. CT-derived muscle mass (SMI) and density (SMD) measurements were combined to form the CT-Sarcopenia score (CT-SS). Thresholds for low SMI and SMD reported by Martin and co-workers and Caan/Xiao and co-workers were combined to form two iterations of the CT-SS. Patients were categorized as normal/high SMI (irrespective of SMD) = 0, low SMI and normal/high SMD = 1 and low SMI and low SMD = 2. The Pearson Chi square test was used to examine the associations between categorical variables and the Chi square test for linear trend was used for ordered variables with multiple categories. Survival data were analysed using univariate and multivariate Cox regression.
Results
One thousand and two patients met the study inclusion criteria. Fifty-five per cent (n = 554) of patients were male and 66% (n = 657) were aged 65 years or older. Twenty-four per cent (n = 240) of patients had TNM stage I disease, 40% (n = 404) stage II and 36% (n = 358) stage III. Eighteen per cent (n = 174) of patients were at risk of malnutrition. Forty-eight per cent (n = 479) of patients had an NLR ≥ 3 and 27% (n = 271) had an mGPS ≥ 1. Similar numbers of patients defined as CT-SS 0, 1 and 2 irrespective of thresholds applied (49%/12%/39% vs. 43%/19%/38%, respectively). Eight hundred and thirty four (n = 834) patients who underwent surgical resection for non-metastatic colorectal cancer with curative intent were alive at 3 years. On univariate analysis, both the CT-SS (Martin/Martin) and CT-SS (Caan/Xaio) were significantly associated with age (P < 0.001 and P < 0.001, respectively), ASA (P < 0.01 and P < 0.001, respectively), MUST (P < 0.001 and P < 0.005, respectively), mGPS (P < 0.001 and P < 0.001, respectively), NLR (P < 0.001 and P < 0.001, respectively), and overall survival (P < 0.001 and P < 0.001, respectively). CT-SS (Caan/Xaio) was significantly associated with TNM stage (P < 0.05), but not CT-SS (Martin/Martin, P = 0.221).
Conclusions
The objective CT-SS was significantly associated with older age, co-morbidity, nutritional risk, systemic inflammation and poorer survival, irrespective of thresholds used. However, the relationship between CT-SS and TNM stage was inconsistent and threshold dependent.
Introduction
The second European Working Group on Sarcopenia in Older People (EWGSOP2) revised operational definition of sarcopenia highlighted the importance of assessing muscle strength, in addition to muscle mass, for defining sarcopenia and its impact on the individual.1 In patients with cancer, a variety of approaches have been used to determine muscle strength including subjective assessments such as performance status to objective assessments such as hand grip strength.2, 3 However, to date, there has been no widely accepted objective assessment to supplement performance status in patients with cancer. Therefore, there is a continued interest in identifying objective assessments of sarcopenia and to determine its impact on clinical outcome in patients with cancer.
CT-derived body composition analysis has facilitated the assessment of skeletal muscle in patients with cancer. Specifically, the quantification of muscle mass and density as part of routine clinical investigations.4, 5 Skeletal muscle mass is most often calculated from the cumulative volume of the intra-abdominal musculature on a CT image slice, generally at the level of the third lumbar vertebra, normalized by the square of the patient's height in metres (skeletal muscle index, SMI), analogous to that of BMI.6 Indeed, CT-derived muscle mass measurements have been shown to be consistent with measurements of other modalities,7 specifically bioelectrical impedance analysis8 and dual-energy X-ray absorptiometry.9 Skeletal muscle density (SMD) on the other hand is an assessment of muscle quality, with striated skeletal muscle, free from fat infiltration, having a greater density on CT analysis compared against that with fat deposition.10, 11 While subject to confounding factors,12 there is now consistent evidence that SMD is associated with physical function in patients with cancer, across a range of cancer subtypes and disease stages.13-15 Therefore, taken together, SMI and SMD, may provide a routine clinical methodology by which sarcopenia and its clinical impact can be characterized.
In isolation, a low SMI and SMD have been negatively associated with clinical outcomes in colorectal cancer.16-18 Furthermore, they have also been associated with prognostic host factors including malnutrition19 and systemic inflammation.20 However, the relationship between CT-derived sarcopenia, malnutrition, systemic inflammation and clinical outcomes in colorectal cancer has yet to be examined. Specifically, whether these measurements together, if carried out using standardized methodology,12 may have complementary prognostic value. Therefore, the aim of the present study was to examine the relationship between CT-derived sarcopenia and clinicopathological characteristics and the relationship with survival in patients undergoing potentially curative surgery for colorectal cancer.
Patients and methods
Patients
Consecutive patients who underwent potentially curative resections for colorectal cancer, within NHS Greater Glasgow and Clyde (NHSGGC), between April 2008 and April 2018, were identified from a prospectively maintained database. Those patients with a pre-operative CT scan, recorded height and weight, pre-operative assessment of the systemic inflammatory response and had TNM stage I–III disease were assessed for inclusion. Exclusion criteria were as follows; patients without satisfactory pre-operative CT imaging, without a recorded height and weight, had no pre-operative assessment of the systemic inflammatory response or had TNM stage IV disease.
Clinicopathological characteristics
Routine demographic details included age, sex and BMI. Age categories were grouped into <64, 65–74, and >74 years. BMI was categorized as <20, 20–24.9, 25–29.9, and ≥30 kg/m2. The Malnutrition Universal Screening Tool (MUST) was used to determine the overall risk of malnutrition. MUST is a three-component score consisting of the patient's current weight status using BMI, unintentional weight loss, and the acute disease effect. Scores were identified retrospectively from patient's medical records. Assessment was made by clinical nursing staff, using a dedicated proforma, within 24 h of admission. Patients were categorized as into low risk (MUST score = 0), medium risk (MUST score = 1), and high risk (score ≥2) of malnutrition, as previously described.21 Patient co-morbidity was classified using the American Society of Anesthesiologists (ASA) grading system: ASA 1, a normal healthy patient; ASA 2, a patient with mild systemic disease; ASA 3, a patient with severe systemic disease that is not incapacitating; and ASA 4, a patient with incapacitating severe systemic disease that is a constant threat to life.22
Tumour site was identified from pre-operative CT imaging, endoscopic and pathology reports. Tumours were staged using the fifth edition of the TNM classification, consistent with practice current in the United Kingdom during the study period.23 Patients were followed-up for a minimum of 3 years following surgery. The date and cause of death were confirmed using hospital electronic case records. Date of last recorded follow-up or last review of electronic case records (1 October 2021), which served as the censor date. Ethical approval was granted by the West of Scotland Research Ethics Committee, Glasgow.
Computed tomography body composition
CT images were obtained at the level of the third lumbar vertebra as previously described.24 Patients with CT imaging taken 3 months or more prior to their surgery were excluded from the study. Furthermore, Scans with significant movement artefact or missing region of interest were not considered for inclusion. Each image was analysed using a free-ware program (NIH Image J version 1.47, http://rsbweb.nih.gov/ij/) shown to provide reliable measurements.25
Region of interest (ROI) measurements were made of subcutaneous fat (SFA), visceral fat (VFA) and skeletal muscle area (SMA) (cm2) using standard Hounsfield Unit (HU) ranges (adipose tissue −190 to −30, and skeletal muscle −29 to +150). These were then normalized for height2 to create indices; subcutaneous fat index (SFI, cm2/m2), and skeletal muscle index (SMI, cm2/m2). Skeletal muscle radiodensity (SMD, HU) was measured from the same ROI used to calculate SMI, as its mean HU.
A high SFI was defined as ≥50.0 cm2/m2 in men and ≥42.0 cm2/m2 in women.26 Visceral obesity was defined as VFA > 160 cm2 for male patients and >80 cm2 for female patients.27 A low SMI was defined as described by Martin et al. as an SMI < 43 cm2/m2 if BMI < 25 kg/m2 and SMI < 53 cm2/m2 if BMI ≥ 25 kg/m2 in male patients and an SMI < 41 cm2/m2 in female patients.4 A low SMI was also described by Caan et al. as an SMI < 52.3 cm2/m2 if BMI < 30 kg/m2 and SMI < 54.3 cm2/m2 if BMI ≥ 30 kg/m2 in male patients and an SMI < 38.6 cm2/m2 if BMI < 30 kg/m2 and SMI < 46.6 cm2/m2 if BMI ≥ 30 kg/m2 in female patients.28 A low SMD was defined by Martin et al. as an SMD < 41 HU in patients with BMI < 25 kg/m2 and <33 HU in patients with BMI > 25 kg/m2.4 A low SMD was also defined by Xiao et al. as <35.5 HU in men and <32.5 HU in women.29
CT-derived muscle measurements (SMI and SMD) were combined to form the CT-sarcopenia score (CT-SS). Patients were categorized as normal/high SMI (irrespective of SMD) = 0, low SMI and normal/high SMD = 1 and low SMI and low SMD = 2. The thresholds for low SMI and SMD reported by Martin and co-workers were combined to form the first iteration of the CT-SS (Martin). Thresholds derived from cohorts of patients with colorectal cancer were also used to calculate a second iteration of the CT-SS for comparison (Caan/Xiao).
Systemic inflammation
Pre-operative haematological and biochemical results were identified from medical records and prospectively recorded. Blood samples were either obtained at pre-operative assessment, within 30 days of surgery, for elective patients or on admission for patients undergoing emergency surgery. An autoanalyser was used to measure serum CRP (mg/L) and albumin (g/L) concentrations (Architect; Abbot Diagnostics, Maidenhead, UK).
Systemic inflammatory status was retrospectively assessed by calculating the neutrophil/lymphocyte ratio (NLR) and modified Glasgow Prognostic Score (mGPS) for each patient, using pre-operative blood results. The NLR was calculated by division of the neutrophil count by the lymphocyte count, obtained from the patient's full blood count (FBC). NLR values were grouped as <3 (considered normal), 3–5 (considered moderate), and >5 (considered raised), as previously described.25 The mGPS was derived as the following: patients with a normal CRP (<10 mg/L) scored 0, those with an elevated CRP (>10 mg/L) alone were scored 1, and those with an elevated CRP (>10 mg/L) and hypoalbuminaemia (<35 g/L) were scored 2, as previously described.30
Statistical analysis
Demographic data, clinicopathological variables, MUST, BMI, CT-SS, SFI, VFA, NLR, and mGPS were presented as categorical variables. The Pearson χ2 test was used to examine the associations between categorical variables, and the χ2 test for linear trend was used for ordered variables with multiple categories.
The relationship between the CT-SS (Martin and Caan/Xiao), clinicopathological characteristics, CT-derived adipose measurements and systemic inflammation were examined using univariate and multivariate binary logistic regression, to calculate OR and 95% CI. Clinicopathological factors that had a P-value <0.1 were taken into a multivariate model using a backward conditional model to identify independently significant factors.
The primary end point was overall survival (OS) at 3 years post operatively. Patients who died within 30-days of surgery were excluded from subsequent survival analysis. The time between the date of surgery and the date of death of any cause was used to define overall survival. Survival data were analysed using univariate and multivariate Cox regression. Those variables associated with a degree of P < 0.1 were entered into a backward conditional multivariate model.
Missing data were excluded from analysis on a variable-by-variable basis. Two-tailed P-values <0.05 were considered statistically significant. Statistical analysis was performed using SPSS software version 25.0. (SPSS Inc., Chicago, IL, USA).
Results
Patient inclusion
The clinicopathological characteristics of the included patients are shown in Table 1 (n = 1002). 55.3% (n = 554) of patients were male and 65.6% (n = 657) were aged 65 years or older. 24% (n = 240) of patients had TNM stage I disease, 40.3% (n = 404) stage II and 35.7% (n = 358) stage III. 17.7% (n = 174) of those with a pre-operative MUST were at risk of malnutrition. The median BMI of the cohort was 27 kg/m2 and 65.1% (n = 652) of patients had a BMI ≥ 25 kg/m2. A high VFA was present in 73% (n = 731) of patients and 80.1% (n = 803) had a high SFI. 47.8% (n = 479) of patients had an NLR ≥ 3 and 27% (n = 271) had an mGPS ≥ 1. In the whole cohort, 83.2% (n = 834) patients who underwent surgical resection for non-metastatic colorectal cancer with curative intent were alive at 3 years. When stratified by site of tumour, 82% (n = 491) of patients with colonic tumours were alive at 3 years post-operatively and 86% (n = 343) of those with rectal tumours.
| Clinicopathological characteristic | n = 1002 (%) |
|---|---|
| Age | |
| <65 | 345 (34.4) |
| 65–74 | 367 (36.6) |
| >74 | 290 (28.9) |
| Sex | |
| Female | 448 (44.7) |
| Male | 554 (55.3) |
| ASA | |
| 1 | 196 (19.6) |
| 2 | 456 (45.5) |
| 3 | 316 (31.5) |
| 4 | 34 (3.4) |
| MUST | |
| Low risk | 810 (80.8) |
| Medium risk | 91 (9.1) |
| High risk | 83 (8.3) |
| Missing | 18 (1.8) |
| Tumour site | |
| Colon | 602 (60.1) |
| Rectum | 400 (39.9) |
| TNM stage | |
| I | 240 (24.0) |
| II | 404 (40.3) |
| III | 358 (35.7) |
| BMI | |
| <20 | 58 (5.8) |
| 20–24.9 | 292 (29.1) |
| 25–29.9 | 337 (33.6) |
| ≥30 | 315 (31.4) |
| High SFI | |
| No | 199 (19.9) |
| Yes | 803 (80.1) |
| High VFA | |
| No | 271 (27.0) |
| Yes | 731 (73.0) |
| Low SMI | |
| No | 495 (49.4) |
| Yes | 507 (50.6) |
| Low SMD | |
| No | 334 (33.3) |
| Yes | 668 (66.7) |
| mGPS | |
| 0 | 731 (73.0) |
| 1 | 109 (10.9) |
| 2 | 162 (16.2) |
| NLR | |
| <3 | 532 (52.2) |
| 3–5 | 310 (30.9) |
| >5 | 169 (16.9) |
| 3 year survival | |
| Yes | 834 (83.2) |
| No | 168 (16.8) |
A low SMI and SMD were present in 51% (n = 507) and 67% of patients (n = 668), respectively, using the Martin thresholds. A low SMI was present in 57% (n = 570) of patients using the Caan thresholds and a low SMD in 58% (n = 584) of patients using the Xiao thresholds. The median (IQR) of SMI for patients categorized as having a low SMI using Martin threshold was 39.1 cm2/m2 (35.5–44.6). The median (IQR) of SMI for patients categorized as having a normal/high SMI using Martin threshold was 51.1 cm2/m2 (45.3–57.6). The median (IQR) of SMI for patients categorized as having a low SMI using Caan threshold was 41.5 cm2/m2 (36.0–47.1). The median (IQR) of SMI for patients categorized as having a normal/high SMI using Caan threshold was 53.4 cm2/m2 (44.5–59.7).
The relationship between SMI, SMD and 3 year survival in patients undergoing potentially curative surgery for CRC using thresholds of Martin and co-workers is shown in Table 2a. In patients with a low SMI, a low SMD was significantly associated with 3 year survival (P < 0.01). The relationship between SMI, SMD and 3 year overall survival in patients undergoing potentially curative surgery, using thresholds of Caan/Xiao and co-workers is shown in Table 2b. In patients with a low SMI, a low SMD was significantly associated with 3 year overall survival (P < 0.001).
| Normal SMI (n = 495) | Low SMI (n = 507) | Total (n = 1002) | P-value | |
|---|---|---|---|---|
| Normal SMD (n = 334) | 190 (88.4%) | 106 (89.1%) | 296 (88.6%) | 0.864 |
| Low SMD (n = 668) | 238 (85.0%) | 300 (77.3%) | 538 (80.5%) | 0.013 |
| Total (n = 1002) | 428 (86.5%) | 406 (80.1%) | 834 (83.2%) | 0.007 |
| P-value | 0.277 | 0.005 | 0.001 |
| Normal SMI (n = 432) | Low SMI (n = 570) | Total (n = 1002) | P-value | |
|---|---|---|---|---|
| Normal SMD (n = 418) | 202 (86.7%) | 165 (89.2%) | 367 (87.8%) | 0.439 |
| Low SMD (n = 584) | 171 (85.9%) | 296 (76.9%) | 467 (80.0%) | 0.010 |
| Total (n = 1002) | 373 (86.3%) | 461 (80.9%) | 834 (83.2%) | 0.022 |
| P-value | 0.817 | <0.001 | 0.001 |
The prevalence of CT-derived sarcopenia scores using thresholds of Martin and co-workers and Caan/Xiao is shown in Table 3. A similar prevalence was observed irrespective of threshold combination used (49%/12%/39% vs. 43%/19%/38%).
| CT-SS 0 (n = %) | CT-SS 1 (n = %) | CT-SS 2 (n = %) | |
|---|---|---|---|
| Martin et al. | 495 (49%) | 119 (12%) | 388 (39%) |
| Caan/Xiao et al. | 432 (43%) | 185 (19%) | 296 (38%) |
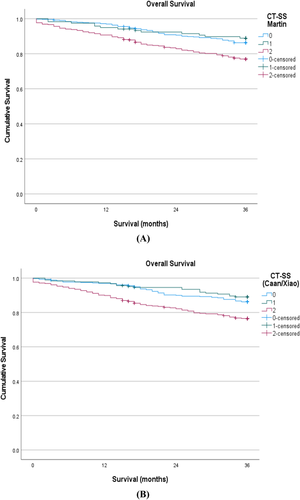
The Kaplan–Meier curve in Figure 1A shows the relationship between the CT-SS (Martin) and overall survival in patients undergoing potentially curative surgery for CRC (log rank P < 0.001). The Kaplan–Meier curve in Figure 1B shows the relationship between CT-SS (Caan/Xiao) and overall survival in patients undergoing potentially curative surgery for CRC (log rank P < 0.001).
The relationship between the CT-SS (Martin and Caan/Xiao), clinicopathological characteristics, CT-derived adipose measurements, systemic inflammation and overall survival in patients undergoing potentially curative surgery for CRC, is shown in Tables 4a and 4b, respectively. On univariate analysis, the CT-SS (Martin) was significantly associated with age (P < 0.001), ASA (P < 0.01), MUST (P < 0.001), tumour site (P < 0.05), BMI (P < 0.001), high SFI (P < 0.05), mGPS (P < 0.001), NLR (P < 0.001), and overall survival (P < 0.001, Table 4a). On univariate analysis, the CT-SS (Caan/Xaio) was significantly associated with age (P < 0.001), sex (P < 0.001), ASA (P < 0.001), MUST (P < 0.05), tumour site (P < 0.05), TNM stage (P < 0.05), BMI (P < 0.001), high SFI (P < 0.05), mGPS (P < 0.001), NLR (P < 0.001), and overall survival (P < 0.001, Table 4b).
| CT-SS 0 (n = 495) | CT-SS 1 (n = 119) | CT-SS 2 (n = 388) | P-valuea | |
|---|---|---|---|---|
| Age | <0.001 | |||
| <65 | 217 (43.8) | 46 (38.7) | 82 (21.1) | |
| 65–74 | 174 (35.2) | 55 (46.2) | 138 (35.6) | |
| >74 | 104 (21.0) | 18 (15.1) | 168 (43.3) | |
| Sex | 0.626 | |||
| Female | 222 (44.8) | 59 (49.6) | 167 (43.0) | |
| Male | 273 (55.2) | 60 (50.4) | 221 (57.0) | |
| ASA | 0.003 | |||
| 1 | 103 (20.8) | 35 (29.4) | 58 (14.9) | |
| 2 | 227 (45.9) | 55 (46.2) | 174 (44.8) | |
| 3 | 155 (31/3) | 27 (22.7) | 134 (34.5) | |
| 4 | 10 (2.0) | 2 (11.9) | 22 (5.7) | |
| MUST | <0.001 | |||
| Low risk | 433 (88.2) | 377 (76.5) | 286 (75.1) | |
| Medium risk | 29 (5.9) | 62 (12.6) | 51 (13.4) | |
| High risk | 29 (5.9) | 54 (11.0) | 44 (11.5) | |
| Missing | 4 | 7 | 7 | |
| Tumour site | 0.015 | |||
| Colon | 281 (56.8) | 69 (58.0) | 252 (64.9) | |
| Rectum | 214 (43.2) | 50 (42.0) | 136 (35.1) | |
| TNM stage | 0.221 | |||
| I | 143 (28.9) | 26 (21.8) | 71 (18.3) | |
| II | 171 (34.5) | 40 (33.6) | 193 (47.9) | |
| III | 181 (36.6) | 53 (44.5) | 124 (32.0) | |
| BMI | <0.001 | |||
| <20 | 17 (3.4) | 7 (5.9) | 34 (8.8) | |
| 20–24.9 | 128 (25.9) | 26 (21.8) | 138 (35.6) | |
| 25–29.9 | 129 (26.1) | 69 (58.0) | 139 (35.8) | |
| ≥30 | 221 (44.6) | 17 (14.3) | 77 (19.8) | |
| High SFI | 0.261 | |||
| No | 87 (17.6) | 33 (27.7) | 79 (20.4) | |
| Yes | 408 (82.4) | 86 (72.3) | 309 (79.6) | |
| High VFA | 0.449 | |||
| No | 124 (25.1) | 42 (35.3) | 105 (27.1) | |
| Yes | 371 (74.9) | 77 (64.7) | 283 (72.9) | |
| mGPS | <0.001 | |||
| 0 | 394 (79.6) | 89 (74.8) | 248 (63.9) | |
| 1 | 56 (11.3) | 17 (14.3) | 36 (9.3) | |
| 2 | 45 (9.1) | 13 (10.9) | 104 (26.8) | |
| NLR | <0.001 | |||
| <3 | 227 (56.0) | 74 (62.2) | 172 (44.3) | |
| 3–5 | 147 (29.7) | 32 (62.9) | 131 (33.8) | |
| >5 | 71 (14.3) | 13 (10.9) | 85 (21.9) | |
| 3 year survival | <0.001 | |||
| Yes | 428 (86.5) | 106 (89.1) | 300 (77.3) | |
| No | 67 (13.5) | 13 (10.9) | 88 (22.7) |
- Each cell is presented as n = (%).
- a P-value is from χ2 analysis.
| CT-SS 0 (n = 432) | CT-SS 1 (n = 185) | CT-SS 2 (n = 385) | P-valuea | |
|---|---|---|---|---|
| Age | <0.001 | |||
| <65 | 203 (47.0) | 69 (37.3) | 73 (19.0) | |
| 65–74 | 152 (35.2) | 77 (42.6) | 138 (35.8) | |
| >74 | 77 (17.8) | 39 (21.1) | 174 (45.2) | |
| Sex | <0.001 | |||
| Female | 237 (54.9) | 83 (44.9) | 128 (33.2) | |
| Male | 195 (45.1) | 102 (55.1) | 257 (66.8) | |
| ASA | <0.001 | |||
| 1 | 96 (22.2) | 47 (25.4) | 53 (13.8) | |
| 2 | 203 (47.0) | 86 (46.5) | 167 (43.4) | |
| 3 | 123 (28.5) | 49 (26.5) | 144 (37.4) | |
| 4 | 10 (2.3) | 3 (1.6) | 21 (5.5) | |
| MUST | 0.036 | |||
| Low risk | 372 (87.1) | 130 (70.3) | 308 (80.0) | |
| Medium risk | 29 (6.8) | 24 (13.0) | 38 (9.9) | |
| High risk | 26 (6.1) | 26 (14.1) | 31 (2.1) | |
| Missing | 5 | 5 | 8 | |
| Tumour site | 0.001 | |||
| Colon | 242 (56.0) | 101 (54.6) | 259 (67.3) | |
| Rectum | 190 (44.0) | 84 (45.4) | 126 (32.7) | |
| TNM stage | 0.033 | |||
| I | 126 (29.2) | 42 (22.7) | 72 (18.7) | |
| II | 152 (35.2) | 80 (43.2) | 172 (44.7) | |
| III | 154 (35.6) | 63 (34.1) | 141 (36.6) | |
| BMI | <0.001 | |||
| <20 | 17 (3.9) | 20 (10.8) | 21 (5.5) | |
| 20–24.9 | 84 (19.4) | 95 (51.4) | 113 (29.4) | |
| 25–29.9 | 151 (35.0) | 47 (25.4) | 139 (36.1) | |
| ≥30 | 180 (41.7) | 23 (12.4) | 112 (29.1) | |
| High SFI | 73 (19.0) | 0.008 | ||
| No | 52 (12.0) | 74 (40.0) | 73 (19.0) | |
| Yes | 380 (88.0) | 111 (60.0) | 312 (81.0) | |
| High VFA | 0.566 | |||
| No | 98 (22.7) | 95 (51.4) | 78 (20.3) | |
| Yes | 334 (77.3) | 90 (48.6) | 307 (79.7) | |
| mGPS | <0.001 | |||
| 0 | 345 (79.9) | 134 (72.4) | 252 (65.5) | |
| 1 | 46 (10.6) | 25 (13.5) | 38 (9.9) | |
| 2 | 41 (9.5) | 26 (14.1) | 95 (24.7) | |
| NLR | <0.001 | |||
| <3 | 254 (58.8) | 89 (48.1) | 180 (46.8) | |
| 3–5 | 128 (29.6) | 64 (34.6) | 118 (30.6) | |
| >5 | 50 (11.6) | 32 (17.3) | 87 (22.6) | |
| 3 year survival | <0.001 | |||
| Yes | 373 (86.3) | 165 (89.2) | 296 (76.9) | |
| No | 59 (13.7) | 20 (10.8) | 89 (23.1) |
- Each cell is presented as n = (%).
- a P-value is from χ2 analysis.
The relationship between the CT-SS (Martin and Caan/Xiao), clinicopathological characteristics, CT-derived adipose measurements, and systemic inflammation in patients undergoing potentially curative surgery for CRC is also shown in Tables 5a and 5b, respectively. On univariate analysis, the CT-SS (Martin) was significantly associated with age (P < 0.001), ASA (P < 0.001), MUST (P < 0.001), tumour site (P < 0.05), BMI (P < 0.001), mGPS (P < 0.001), and NLR (P < 0.001). Age (P < 0.001), BMI (P < 0.001), and mGPS (P < 0.001) remained significantly associated with CT-SS (Martin) on multivariate analysis. On univariate analysis, the CT-SS (Caan/Xiao) was significantly associated with age (P < 0.001), sex (P < 0.001), ASA (P < 0.001), tumour site (P < 0.001), TNM stage (P < 0.05), mGPS (P < 0.001), and NLR (P < 0.001). Age (P < 0.001), sex (P < 0.001), and mGPS (P < 0.001) remained significantly associated with CT-SS (Caan/Xiao) on multivariate analysis.
| OR (univariate) | P-valuea | OR (multivariate) | P-valuea | |
|---|---|---|---|---|
| Age (<55/65–74/>74) | 2.11 (1.78–2.50) | <0.001 | 1.99 (1.66–2.38) | <0.001 |
| Sex (male/female) | 1.12 (0.86–1.44) | 0.389 | - | - |
| ASA (1/2/3/4) | 1.37 (1.14–1.64) | <0.001 | - | 0.228 |
| MUST (Low/medium/high risk) | 1.58 (1.28–1.95) | <0.001 | - | 0.716 |
| Tumour site (Colon/rectum) | 0.72 (0.55–0.93) | 0.012 | - | 0.762 |
| BMI (<20/20–24.9/35–29.9/>30 kg/m2) | 0.62 (0.54–0.71) | <0.001 | 0.67 (0.57–0.78) | <0.001 |
| mGPS (0/1/2) | 1.74 (1.47–2.10) | <0.001 | 1.60 (1.33–1.93) | <0.001 |
| NLR (<3/3–5/>5) | 1.45 (1.22–1.71) | <0.001 | - | 0.082 |
- a P-value is from binary logistics regression analysis.
| OR (univariate) | P-valuea | OR (multivariate) | P-valuea | |
|---|---|---|---|---|
| Age (<55/65–74/>74) | 2.37 (1.99–2.82) | <0.001 | 2.38 (1.99–2.86) | <0.001 |
| Sex (male/female) | 2.16 (1.66–2.82) | <0.001 | 2.32 (1.75–3.10) | <0.001 |
| ASA (1/2/3/4) | 1.53 (1.30–1.81) | <0.001 | - | 0.220 |
| MUST (Low/medium/high risk) | 1.02 (0.82–1.26) | 0.867 | - | - |
| Tumour site (colon/rectum) | 0.61 (0.47–0.79) | <0.001 | - | 0.164 |
| TNM stage (I/II/III) | 1.19 (1.00–1.41) | 0.044 | - | 0.056 |
| BMI (<20/20–24.9/35–29.9/>30 kg/m2) | 0.96 (0.84–1.11) | 0.604 | - | - |
| High SFI (yes/no) | 1.10 (0.80–1.51) | 0.573 | - | - |
| mGPS (0/1/2) | 1.56 (1.32–1.85) | <0.001 | 1.42 (1.18–1.71) | <0.001 |
| NLR (<3/3–5/>5) | 1.37 (1.16–1.63) | <0.001 | - | 0.079 |
- a P-value is from binary logistics regression analysis.
The relationship between clinicopathological characteristics, BMI, CT-derived adipose measurements, CT sarcopenia score (CT-SS Martin), systemic inflammation and overall survival in patients undergoing potentially curative surgery for CRC is shown in Table 6a. On univariate analysis, age (P < 0.001), ASA (P < 0.001), MUST (P < 0.001), TNM stage (P < 0.001), BMI (P < 0.001), CT-SS (P < 0.001), mGPS (P < 0.001), and NLR (P < 0.001) were significantly associated with overall survival. On multivariate analysis, ASA (P < 0.001), MUST (P < 0.001), TNM stage (P < 0.001), mGPS (P < 0.05), and NLR (P < 0.05) remained significantly associated with overall survival (see Table 6a).
| HR (univariate) | P-valuea | HR (multivariate) | P-valuea | |
|---|---|---|---|---|
| Age | 1.46 (1.19–1.77) | <0.001 | - | 0.291 |
| Sex | 1.21 (0.89–1.64) | 0.232 | - | - |
| ASA | 1.68 (1.37–2.05) | <0.001 | 1.56 (1.27–2.13) | <0.001 |
| MUST | 2.07 (1.72–2.48) | <0.001 | 1.43 (1.24–1.64) | <0.001 |
| Tumour site | 0.75 (0.55–1.04) | 0.080 | - | 0.445 |
| TNM stage | 1.65 (1.33–2.04) | <0.001 | 1.55 (1.24–1.95) | <0.001 |
| BMI | 0.71 (0.60–8.34) | <0.001 | - | 0.342 |
| High SFI | 0.77 (0.54–1.10) | 0.148 | - | - |
| High VFA | 0.85 (0.61–1.19) | 0.346 | - | - |
| CT sarcopenia score (0/1/2) | 1.36 (1.15–1.60) | <0.001 | - | 0.334 |
| mGPS | 1.55 (1.30–1.84) | <0.001 | 1.26 (1.05–1.52) | 0.015 |
| NLR | 1.46 (1.21–1.77) | <0.001 | 1.24 (1.02–1.51) | 0.035 |
- a P-value is from univariate and multivariate Cox regression analysis.
The relationship between clinicopathological characteristics, CT-derived adipose measurements, CT sarcopenia score (CT-SS Martin), systemic inflammation and overall survival in patients undergoing potentially curative surgery for CRC, without the inclusion of BMI, is shown in Table 6b. On univariate analysis, age (P < 0.001), ASA (P < 0.001), MUST (P < 0.001), TNM stage (P < 0.001), CT-SS (P < 0.001), mGPS (P < 0.001), and NLR (P < 0.001) were significantly associated with overall survival. On multivariate analysis, ASA (P < 0.05), MUST (P < 0.001), TNM stage (P < 0.001), mGPS (P < 0.05), and NLR (P < 0.05) remained significantly associated with overall survival (see Table 6b).
| HR (univariate) | P-valuea | HR (multivariate) | P-valuea | |
|---|---|---|---|---|
| Age | 1.46 (1.19–1.77) | <0.001 | - | 0.194 |
| Sex | 1.21 (0.89–1.64) | 0.232 | - | - |
| ASA | 1.68 (1.37–2.05) | <0.001 | 1.48 (1.17–1.86) | 0.001 |
| MUST | 2.07 (1.72–2.48) | <0.001 | 1.79 (1.48–2.17) | <0.001 |
| Tumour site | 0.75 (0.55–1.04) | 0.080 | - | 0.809 |
| TNM stage | 1.65 (1.33–2.04) | <0.001 | 1.54 (1.23–1.93) | <0.001 |
| High SFI | 0.77 (0.54–1.10) | 0.148 | - | - |
| High VFA | 0.85 (0.61–1.19) | 0.346 | - | - |
| CT sarcopenia score (0/1/2) | 1.36 (1.15–1.60) | <0.001 | - | 0.227 |
| mGPS | 1.55 (1.30–1.84) | <0.001 | 1.26 (1.05–1.52) | 0.014 |
| NLR | 1.46 (1.21–1.77) | <0.001 | 1.25 (1.03–1.53) | 0.027 |
- a P-value is from univariate and multivariate cox regression analysis.
The relationship between clinicopathological characteristics, BMI, CT-derived adipose measurements, CT sarcopenia score (CT-SS Cann/Xiao), systemic inflammation and overall survival in patients undergoing potentially curative surgery for CRC is shown in Table 7a. On univariate analysis, CT-SS was significantly associated with overall survival (P < 0.001). On multivariate analysis, ASA (P < 0.001), MUST (P < 0.001), TNM stage (P < 0.001), and mGPS (P < 0.05) remained significantly associated with overall survival (See Table 7a).
| HR (univariate) | P-valuea | HR (multivariate) | P-valuea | |
|---|---|---|---|---|
| Age | 1.46 (1.19–1.77) | <0.001 | - | 0.293 |
| Sex | 1.21 (0.89–1.64) | 0.232 | - | - |
| ASA | 1.68 (1.37–2.05) | <0.001 | 1.43 (1.14–1.81) | <0.001 |
| MUST | 2.07 (1.72–2.48) | <0.001 | 1.80 (1.49–2.17) | <0.001 |
| Tumour site | 0.75 (0.55–1.04) | 0.080 | - | 0.521 |
| TNM stage | 1.65 (1.33–2.04) | <0.001 | 1.52 (1.21–1.91) | <0.001 |
| BMI | 0.71 (0.60–8.34) | <0.001 | - | 0.362 |
| High SFI | 0.77 (0.54–1.10) | 0.148 | - | - |
| High VFA | 0.85 (0.61–1.19) | 0.346 | - | - |
| CT sarcopenia score (0/1/2) | 1.39 (1.17–1.64) | <0.001 | - | 0.077 |
| mGPS | 1.55 (1.30–1.84) | <0.001 | 1.22 (1.01–1.48) | 0.040 |
| NLR | 1.46 (1.21–1.77) | <0.001 | - | 0.052 |
- a P-value is from univariate and multivariate Cox regression analysis.
The relationship between clinicopathological characteristics, CT-derived adipose measurements, CT sarcopenia score (CT-SS Caan/Xiao), systemic inflammation and overall survival in patients undergoing potentially curative surgery for CRC, without the inclusion of BMI, is shown in Table 7b. On multivariate analysis, ASA (P < 0.001), MUST (P < 0.001), TNM stage (P < 0.001), and mGPS (P < 0.05) remained significantly associated with overall survival (see Table 7b).
| HR (univariate) | P-valuea | HR (multivariate) | P-valuea | |
|---|---|---|---|---|
| Age | 1.46 (1.19–1.77) | <0.001 | - | 0.376 |
| Sex | 1.21 (0.89–1.64) | 0.232 | - | - |
| ASA | 1.68 (1.37–2.05) | <0.001 | 1.53 (1.24–1.88) | <0.001 |
| MUST | 2.07 (1.72–2.48) | <0.001 | 1.77 (1.46–2.14) | <0.001 |
| Tumour site | 0.75 (0.55–1.04) | 0.080 | - | 0.442 |
| TNM stage | 1.65 (1.33–2.04) | <0.001 | 1.54 (1.22–1.93) | <0.001 |
| High SFI | 0.77 (0.54–1.10) | 0.148 | - | - |
| High VFA | 0.85 (0.61–1.19) | 0.346 | - | - |
| CT sarcopenia score (0/1/2) | 1.39 (1.17–1.64) | <0.001 | - | 0.077 |
| mGPS | 1.55 (1.30–1.84) | <0.001 | 1.22 (1.01–1.48) | 0.040 |
| NLR | 1.46 (1.21–1.77) | <0.001 | - | 0.052 |
- a P-value is from univariate and multivariate Cox regression analysis.
Discussion
The results of the present study showed that in a large cohort of patients with operable colorectal cancer, the combination of a low muscle mass and density (CT-sarcopenia score, CT-SS) was significantly associated with older age, greater co-morbidity and nutritional risk, systemic inflammation and poorer survival, irrespective of threshold used to define a low SMI or SMD. Therefore, this simple objective score has clinical utility to inform on likely outcome and may be useful in the investigation of the underlying mechanisms of sarcopenia in patients with cancer.
CT-derived skeletal muscle mass and density measurements have been shown to have independent prognostic value in patients with cancer.31, 32 However, the present study found that patients with both a low skeletal muscle mass and density had significantly reduced overall survival compared to patients with a normal muscle mass or density. These results suggest that the prognostic value of CT-derived muscle measurements is likely to be greatest when used in combination, such as the proposed CT-SS. Combining CT-derived muscle measurements has previously been proposed in the literature in the form of the unvalidated skeletal muscle gauge (SMG), the product of SMI × SMD. However, the methodology used did not account for the relative importance and accuracy of the individual components. In contrast, the proposed CT-SS is based on the measurement of SMI using standardized thresholds and methodology validated against other techniques.7 Furthermore, the CT-SS utilizes standardized thresholds for SMD and accounts for potential confounding factors in the methodology, such as the phase of the contrast enhanced CT scan.12, 33 Therefore, the CT-SS reflects an incremental approach to defining sarcopenia and the impact on clinical outcomes.
The association between CT-derived sarcopenia and TNM stage was found to be inconsistent and threshold dependent in the present study (see Tables 4a and 4b). These results may suggest that surveillance of muscularity should occur across the different stages of the patient trajectory and even at the earlier TNM stages. As such, the present observations are in keeping with those of a recent review by our group, of 160 studies of CT-derived SMI and SMD that found poor muscle status was endemic in patients with cancer, suggesting that tumour stage was not a major determinant of sarcopenia.6 Furthermore, in the review we also hypothesized that the prognostic value of sarcopenia may reflect its measure of the nutritional and functional reserve of the cancer patient and that it is imperative that such body composition measurements be used in conjunction with potential confounding factors such as systemic inflammation.6 Hacker and co-workers reported similar observations in a recent longitudinal study of patients with advanced gastric and oesophagogastric junctional cancer.34 Therefore, taken together, the studies support this hypothesis and that sarcopenia measurements (such as the CT-SS) should be used in conjunction with measures of systemic inflammation.
In the present cohort, a low skeletal muscle mass and density were found to be prevalent across disease stages, on a background of CT-derived obesity, in patients with colorectal cancer. These observations are consistent with recent work by Martin and co-workers who reported that a low skeletal muscle mass and density were endemic in an obese population, across a range histological subtypes and disease stages.35 However, it was of interest that neither of the CT-derived measures of adiposity such as SFI and VFA had prognostic value. Furthermore, neither the presence of a high SFI or high VFA could further stratify survival in patients who had CT-derived sarcopenic (CT-SS ≥ 1, P = 0.209 and P = 0.674, respectively). Therefore, the present results are consistent with the work of Caan and co-workers.28
There are a number of limitations to the present study. Firstly, this is a single centre study and may be subject to bias. However, the present observations were made using routine clinical available measures and should be readily validated. Lastly, CT analysis of SMD has been shown to be dependent on methodology, such contrast enhancement.36 However, in the present study only patients who underwent CT-imaging with intravenous contrast were included in the analysis.
In conclusion, CT-derived muscle measurements, SMI and SMD, when used in combination have additional prognostic value in patients with colorectal cancer. The objective CT-SS was significantly associated with older age, co-morbidity, nutritional risk, systemic inflammation and poorer survival, irrespective of thresholds used.
Funding
This study was funded by the Academic Unit of Surgery, University of Glasgow.
Conflict of interest
None declared.




