Does Fusobacterium in Colorectal Cancer Sites Originate From the Oral Cavity? A Pilot Study
ABSTRACT
Objectives
Fusobacterium can contribute to oral diseases, but also pose as a systemic risk factor. This genus, and especially F. nucleatum, can be found in colorectal cancer (CRC) tissue and is involved in multiple aspects of this type of cancer. Previous studies indicated a possible oral origin of these bacteria; however, stronger evidence is needed to reach a definitive conclusion. This pilot study aimed to establish a method to successfully compare, at the strain level, fusobacteria from the oral cavity and CRC resection material for future cohort studies of CRC patients.
Material and Methods
In a first cohort of eight periodontitis patients, gingival crevicular fluid and saliva were collected. Fusobacterium was isolated on two different media. In a second cohort, saliva and CRC resection material were collected from ten CRC patients. These samples were used for screening of Fusobacterium with culturing, 16S rRNA gene profiling and a PCR-based approach.
Results
In the first cohort, different Fusobacterium species were identified in GCF and saliva samples. However, as the total yield of Fusobacterium seemed slightly higher in saliva samples, it was therefore preferred for subsequent sample collection. Thus, in the second cohort, patient-matched saliva and CRC resection material were screened for Fusobacterium and this showed that nine patients were culture-positive in the saliva samples; however, no Fusobacterium could be isolated from the resection material. On the other hand, 16S rRNA gene profiling of the resection material indicated that eight CRC patients were positive for Fusobacterium. All eight of these patients carried Fusobacterium in their saliva, indicated by both marker gene PCR and culture-based screening.
Conclusions
These pilot results are compatible with data from previous studies, indicating a possible link between oral and CRC-associated Fusobacterium, and a more in-depth analysis of specific strains and their characteristics in a larger cohort is justified.
Trial Registration
The protocol was registered at clinicaltrials.gov (NCT05945082).
1 Introduction
Colorectal cancer (CRC) ranks fifth in terms of global incidence and fourth in terms of the mortality rate compared to other cancer types (Sung et al. 2021). Genomic studies have provided the earliest evidence that bacteria are prevalent in CRC tumor tissue; especially Fusobacterium nucleatum has been associated with CRC, although other fusobacteria also reside in the colon (Castellarin et al. 2012; Kostic et al. 2012; Richardson et al. 2020). Later reports indicated that this bacterium is involved in multiple aspects of CRC development and progression (Yu, Kim, and Park 2020; Yang et al. 2017; Rubinstein et al. 2019; Kostic et al. 2013; Gur et al. 2019; Casasanta et al. 2020; Chen et al. 2020; Zhang et al. 2019; Yu et al. 2017). The tumor progression is accelerated by Fusobacterium-mediated activation of the epidermal growth factor receptor (EGFR) signaling pathways, which stimulates the epithelial–mesenchymal transition of tumor cells (Yu, Kim, and Park 2020), and Fusobacterium also accelerates proliferation of CRC cells by Toll-like receptor 4 (TLR4)-activated NF-κB signaling (Yang et al. 2017) and activation of the Wnt/β-catenin pathway (Rubinstein et al. 2019). Additionally, Fusobacterium selectively recruits tumor-infiltrating myeloid cells that generate a pro-inflammatory microenvironment (Kostic et al. 2013) and prevents killing of CRC cells by inhibition of natural killer cells and tumor-infiltrating T-cells (Gur et al. 2019). Fusobacterium also drives metastasis of CRC cells (Casasanta et al. 2020; Chen et al. 2020). Finally, Fusobacterium makes CRC cells more resistant to chemotherapy due to modulation of the host autophagy and active inhibition of apoptosis, both through activation of the TLR4/NF-κB signaling pathway (Zhang et al. 2019; Yu et al. 2017). Taking all this into account, it is not surprising that the prevalence and abundance of Fusobacterium in CRC tumor tissue have been associated with an overall poorer clinical outcome (Mima et al. 2016); nevertheless, controversial results (i.e., better CRC treatment outcomes) have also been reported (Alexander et al. 2023).
Fusobacterium is a genus of obligate anaerobic and gram-negative bacteria that tend to form filamentous rods with tapered ends. It is a diverse genus, consisting of several species and subspecies, of which most are commonly found in the oral cavity and are among the highest abundant members of the oral microbiota (Socransky et al. 1998; Motoc et al. 2023). Although they are commensal bacteria, fusobacteria are associated with periodontitis, being crucial in the formation of subgingival biofilm (Socransky et al. 1998; Zijnge et al. 2010, 2012; Ximénez-Fyvie, Haffajee, and Socransky 2000).
Poor oral health is an important global problem, especially in adults (Peres et al. 2019). In 2019, over 3.5 billion people were suffering from an oral disorder according to the Global Burden of Disease study, corresponding to approximately 45% of the global population (Global Burden of Disease Collaborative Network 2020). Although oral diseases, such as dental caries and periodontitis, can have a major impact within the oral cavity (e.g., tooth loss), they also represent a substantial systemic risk factor. Specifically, periodontitis, a chronic inflammatory disease of the tooth-supporting structures caused by dysbiosis in a susceptible host (Lamont, Koo, and Hajishengallis 2018), has been linked to the development, progression, and severity of several systemic, mostly inflammation-driven diseases. This link between periodontitis and systemic disorders is primarily based on two pathways, that is, via dissemination of oral or periodontal pathogens and due to the chronic inflammatory burden (Hajishengallis 2022). Periodontitis has been associated, among other diseases, with diabetes mellitus (Borgnakke 2019; Graziani et al. 2018), cardiovascular diseases (Sanz et al. 2020), autoimmune disease (Rodríguez-Lozano et al. 2019; Hajishengallis and Chavakis 2021), inflammatory bowel diseases (Bertl et al. 2022; Madsen et al. 2023; Domokos et al. 2022), and various cancers, including CRC (Fu et al. 2022; Li et al. 2021).
Interestingly, recent research based on only three positive patient-matched samples has hinted that CRC-associated fusobacteria may originate from the oral cavity, most likely translocating via the hematogenous route and thus highlighting the importance of oral health (Abed et al. 2020). To overcome the difficulties in the isolation of fusobacteria from CRC material, more recently, whole-genome sequencing and a PCR product of the hypervariable clustered regularly interspaced short palindromic repeats (CRISPR)-associated regions were used (Shimomura et al. 2023). In line with previous results, these data also indicated a possible oral origin of CRC-associated fusobacteria; however, only a small sample size was used for whole-genome sequencing (i.e., five patient-matched samples), whereas limited marker genes instead of the whole genome were analyzed for the remaining samples that were not sequenced.
Therefore, the aim of this pilot study was to establish a method to successfully compare fusobacteria at the strain level from the oral cavity and CRC resection material, as it is expected that this genus is present in both locations and CRC-associated Fusobacterium might have an oral origin. To this end, after comparing different oral sampling methods, patient-matched saliva samples and CRC resection material were used to detect different Fusobacterium species with either a culture-based approach, 16S rRNA gene profiling, or marker gene PCR.
2 Methods
2.1 Patient Population
Two different patient populations were included. The first population (n = 8; “periodontitis patients”) included patients diagnosed with periodontitis Stage 3 or 4 (Tonetti, Greenwell, and Kornman 2018). These patients were visiting the Department of Periodontology (Faculty of Odontology, University of Malmö, Sweden) for regular supportive periodontal care and had at least one tooth with a probing pocket depth of ≥ 5 mm. The reason why periodontitis patients were chosen for this first population was to collect oral samples from a patient group with high chances of harboring a sufficient amount of F. nucleatum to simplify the decision process on the appropriate oral sampling method, but not to relate the results to the periodontal status of these patients. The second population (n = 10; “CRC patients”) included patients who were diagnosed with CRC and scheduled for surgical treatment at the Skåne University Hospital (Malmö, Sweden). Gender, age, and smoking status were recorded for both populations, whereas cancer diagnosis and localization and intake of antibiotics in the preceding 3 months were additionally recorded in the second population (Table 1). The protocol was approved by the regional ethical review board (Dnr. 2016/469) and registered at clinicaltrials.gov (NCT05945082).
| Patient | Gender Smoking | Age BMI | Diagnosis Type | Localization | Intake of systemic antibiotics (in the preceding 3 months) | Comorbidities |
|---|---|---|---|---|---|---|
| 9 | m Never |
67.4 20.4 |
Cancer Adenocarcinoma |
Left, rectal | Yes | Chronic lymphocytic leukemia |
| 10 | m Never |
86.4 25.8 |
Cancer Adenocarcinoma |
Left | Yes | Hypertension |
| 11 | f Never |
90.9 28.2 |
Not cancer Adenoma, low-grade dysplasia |
Right | Yes | Hypertension, pacemaker, pulmonary embolism |
| 12 | m Never |
65.1 26.4 |
Cancer Adenocarcinoma |
Left | No | Hypertension |
| 13 | m Former |
86.7 25.8 |
Cancer Adenocarcinoma |
Left | No | Atrial flutter, cerebral vascular insult, acute myocardial infarction, macroglobulinemia |
| 14 | m Current |
78.5 32.4 |
Cancer Adenocarcinoma |
Left, rectal | No | Atrial flutter, hypertension, chronic obstructive lung disease, benign prostata hyperplasia |
| 15 | f Never |
76.7 20.0 |
Cancer Adenocarcinoma |
Left, rectal | No | Breast cancer, hypertension |
| 16 | m Former |
68.0 26.6 |
Cancer Adenocarcinoma |
Right, appendix | Yes | Goiter, psoriasis |
| 17a | f Former |
80.9 23.2 |
Cancer Adenocarcinoma |
Left | Yes | Hypertension, deep vein thrombosis |
| 18 | f Never |
77.5 21.8 |
Cancer Adenocarcinoma |
Left | No | Hypertension, diabetes mellitus, atrial flutter |
- Abbreviations: BMI, body mass index score; f, female; m, male.
- a Re-treatment; first surgery for cancer 12 years ago.
2.2 Sampling of Gingival Crevicular Fluid (GCF) and Saliva in the Periodontitis Patients
In each patient, one tooth with a probing pocket depth of ≥ 5 mm was chosen randomly for subgingival microbiological sampling by collecting GCF with paper points. The selected tooth was protected from contamination with saliva and supragingival plaque was carefully removed. One sterile paper point (ISO 55) was inserted into the bottom of the pocket and kept in place for 30 s. This process was repeated at the same sampling site three times, that is, in total, four paper points were collected per sampling site. All paper points of the same sampling site were pooled and stored in 1 mL of eSwab Amies medium (Copan Group, Brescia, Italy) mixed with 3 mL of 20% glycerol at −80°C until analysis. For saliva sampling, a regular-sized, pre-packed FLOQSwab (Copan Group) was used to collect unstimulated, whole saliva. Although for the first two patients the saliva was collected only from the back of the tongue, for the next six patients, the saliva was collected from the cheeks, vestibulum, the floor of the mouth, the back of the tongue, and the palate to ensure that a sufficient amount of saliva was obtained. After collection, the swab was stored in 1 mL of eSwab Amies medium mixed with 3 mL of 20% glycerol at −80°C until analysis.
2.3 Sampling of Saliva and Resection Material in the CRC Patients
The saliva sampling in the CRC patients was performed with a swab from the cheeks, vestibulum, the floor of the mouth, the back of the tongue, and the palate, which was stored in 1 mL of eSwab Amies medium with 20% glycerol at −80°C until analysis. All saliva collections were performed maximum 24 h before surgical CRC treatment. After surgical resection of the colorectal tumor, one biopsy of approximately 3 × 3 × 3 mm from the tumor site was collected from each patient and stored in the same medium and temperature.
2.4 Sample Preparation Before Inoculation of Bacterial Media
Before inoculation of the media, CRC resection material was cut into smaller pieces and 0.25 g was homogenized in 200 µL of Amies medium (Copan Group) with 0.1 g of zirconium beads (0.55 mm; Biospec products, Bartlesville, USA) and one glass bead (3 mm, Biospec products) in a homogenizer (Precellys 24, Bertin Technology, Montigny-le-Bretonneux, France) at three cycles of 4500 RPM for 45 s with intervals of 45 s. After this, the samples were centrifuged at 3000 RPM for 1 min and the supernatant was used for bacterial isolation. All saliva samples were homogenized by vortexing before inoculation.
2.5 Isolation of Fusobacterium Species From GCF and Saliva Samples and CRC Resection Material
For the isolation of Fusobacterium from all samples, Brucella blood agar (Mediaproducts BV, Groningen, the Netherlands) and Fusobacterium-specific medium (Fastidious anaerobe agar containing 3 mg/L josamycine, 1 mg/L norfloxacin, 4 mg/L vancomycin, and 5% defibrinated horse blood; Mediaproducts BV) were used and reduced overnight before the experiments. Fifty microliters of either the GCF or saliva samples or the CRC resection lysates was streaked into three segments per agar medium. After inoculation, the plates were incubated for 2 days under anaerobic conditions (10% H2, 10% CO2, and 80% N2) and at 37°C in a Whitley A35 Workstation (Don Whitley Scientific Ltd, Bingley, UK) and pure colonies were obtained using a classical pure culture technique that involved at least three rounds of streaking until single colonies were obtained, whose preliminary purity was assessed using Gram staining afterward.
2.6 Bacterial Identification and Storage
The identity of the bacterial isolates was assessed by matrix-assisted laser desorption/ionization coupled to a time-of-flight mass spectrometer (MALDI-TOF MS; Biotyper Microflex, Bruker Daltonics, Billerica, USA; database version 11) as described previously (Veloo et al. 2016). In short, single colonies were spotted on a polished stainless-steel MALDI target. Air-dried spots were covered with 1 µL of 2-Cyano-3-(4-hydroxyphenyl) acrylic acid matrix (10 mg/mL) and air-dried again. Subsequently, the spotted isolates were analyzed by summing 240 measured shots, using the Biotyper Microflex (Veloo et al. 2016; Almuzara et al. 2016). Identification of the isolates was considered reliable to the species level if results had a log-score of 2.0 or higher, between 1.7 and 1.99 for genus identification, and not reliable if lower than 1.7, as recommended by the manufacturer (Bruker Daltonics). All isolates that were reliably identified as Fusobacterium were stored at −80°C in a 1 mL mix of Brain–Heart infusion broth (Oxoid Ltd, Hampshire, UK) and 85% (v/v) glycerol at a ratio of 1:4 until further use.
2.7 Next-Generation 16S rRNA Gene Amplicon Sequencing of CRC Resection Material
DNA from 0.25 g of the CRC resection material was extracted using a repeated bead-beating method as described previously (de Goffau et al. 2013). The sequencing of the samples was performed according to previously described methods (Heida et al. 2016). In short, amplification was performed with PCR using modified 314 F and 806 R primers, targeting the V3–V4 region of the 16S rRNA gene. The samples were barcoded with six nucleotides added to the reverse primer. An in-house Illumina MiSeq was used to sequence the samples on a 2 × 300 bp cartridge (Illumina Inc., San Diego, USA). Afterward, the reads were joined, quality control was performed (1% error rate), and the primers were cut using VSEARCH (Rognes et al. 2016). The denoising of the reads, chimeric read removal, singleton removal, and dereplication were performed using both VSEARCH and USEARCH (Rognes et al. 2016; Edgar 2010). The amplicon sequence variants (ASVs) were annotated using the Ribosomal Database Project (RDP) classifier based on the RDP set 18 (Cole et al. 2014; Wang et al. 2007). The relative abundance of the ASVs was calculated as the read count of each ASV divided by the total read count of the mapped reads after rarefaction to the same depth. The ASVs that were annotated as Fusobacterium were compared with reference 16S rRNA gene sequences of known species within the genus. All reference sequences were obtained from NCBI. The Fusobacterium ASVs and the reference sequences were aligned using MUSCLE (Edgar 2004). The determination of the phylogenetic relationship was based on the nucleotide substitution rates after elimination of all positions with gaps or missing data. The data were visualized with a phylogenetic tree that was constructed based on evolutionary distances of the maximum likelihood method (Tamura–Nei model) (Felsenstein 1981; Tamura and Nei 1993) using Mega software (version X) (Kumar et al. 2018). The confidence in topology of the tree was estimated with bootstrap values, which were calculated based on 1000 sampling replications (Felsenstein 1985).
2.8 PCR Amplification of a Fusobacterium-Specific Conserved 16S rRNA Gene Region From Saliva Samples
The DNA from 0.25 g of the saliva samples was extracted using the same method, but the isopropanol in the precipitation step was omitted. To detect the presence of Fusobacterium, a genus-specific conserved region of the 16S rRNA gene was amplified using the previously described PCR primers FUSO1 (forward primer: 5′-GAG AGA GCT TTG CGT CC-3′) and FUSO2 (reverse primer: 5′-TGG GCG CTG AGG TTC GAC-3′) (Nagano et al. 2007). Each PCR reaction consisted of 50% v/v 2X Phire Hot Start II PCR Master Mix (Thermo Scientific, Waltham, USA), 0.2 µM of each primer, and 37.5 ng of DNA dissolved in nuclease-free water, resulting in an end volume of 25 µL. A program was run consisting of 1x denaturation at 98°C for 30 s, followed by 35 cycles of denaturation (98°C, 10 s), annealing (60°C, 10 s), and extension (72°C, 15 s), ending after these cycles with a final step of incubation at 72°C for 2 min. The final PCR products were separated with gel electrophoresis on a 1% agarose gel in 1x TBE buffer. All samples and the DNA ladder (FastRuler Low Range DNA Ladder, Thermo Scientific) were loaded with 6x MassRuler DNA Loading Dye (Thermo Scientific) and Midori Green advance DNA stain (Nippon Genetics). The loaded gel was run at 150 V for 30 min. Afterward, the DNA was visualized using a gel imaging system (Gel Doc EZ imager, BioRad, Hercules, USA).
3 Results
3.1 Mode of Sampling From the Oral Cavity Yielded Comparable Number of Different Cultured Fusobacterium Species
Among the periodontitis patients, there were three males and five females, aged between 34 and 72 years, two never smokers, two former smokers, and four current smokers. Figure 1 shows that GCF sampling resulted in the isolation of F. nucleatum in six samples, whereas five saliva samples were positive for this bacterium; except for patient two, the positive saliva samples were in agreement with the GCF samples. Interestingly, cultivation from GCF samples resulted in the isolation of F. naviforme, which could not be isolated from saliva samples. On the other hand, F. periodonticum was exclusively cultured from saliva samples, whereas GCF samples were culture-negative for this species. Although the mode of sampling resulted in different Fusobacterium species, the total yield of this genus seemed slightly higher in saliva samples. Due to this and as collection of saliva with swabs from the oral cavity is easier and less prone to contamination, it was decided to move forward with this sampling method for further collection.
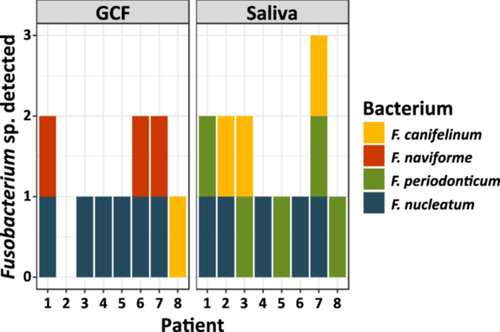
3.2 Targeted Culturing Showed That Different Fusobacterium Species Were Highly Present in the Oral Cavity of CRC Patients, But Could Not be Cultured From CRC Resection Material
All CRC patients, except Patient 15, were culture-positive for the genus Fusobacterium in the saliva samples. The species F. nucleatum was isolated in all culture-positive patients (Figure 2). In 55.6% of all culture-positive patients, F. periodonticum was found to be the second most abundant species within this genus. On the other hand, only one patient was culture-positive for F. naviforme, a species that is also associated with infections in gingival tissue (Bao et al. 2020), and one patient was culture-positive for other Fusobacterium species that could not be identified from our MALDI-TOF MS database (Figure 2). Isolation of Fusobacterium from the CRC resection material by culturing was not successful (data not shown).
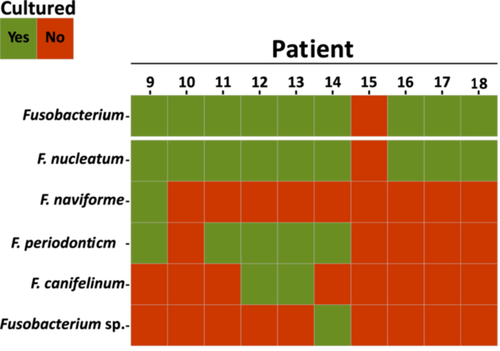
3.3 16S rRNA Gene Amplicon Sequencing Revealed Presence of Fusobacterium in CRC Resection Material and Phylogenetic Analysis of the ASVs Indicated the Presence of Multiple Species in the Tumor Site
As Fusobacterium was not successfully isolated from the CRC resection material by culturing, the bacterial communities were characterized based on 16S rRNA gene amplicon sequencing. Most bacterial genera in the samples belonged to the Firmicutes phylum (Figure 3; 45.6%−74.8%). This phylum was previously reported to be among the most abundant in the human gut microbiota (Gacesa et al. 2022; Zhernakova et al. 2016; Turnbaugh et al. 2007). Interestingly, most of the samples were colonized with the phylum Fusobacteria, of which Fusobacterium is the only represented genus in our data set (Table S1). Eight out of the ten samples were positive for this genus, except for patients 12 and 15 (Figure 3). The relative abundance of this genus ranged between 0.1% (Patient 16) and 2.9% (Patient 17) in the patients who were positive.
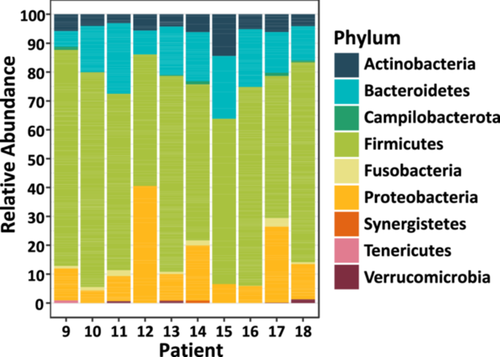
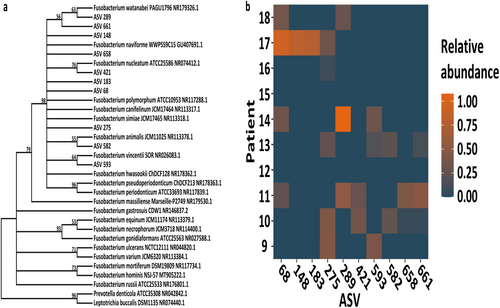
Most saliva samples were culture-positive with certain species of Fusobacterium; therefore, evolutionary analysis was performed on the ASVs that were found in the resection material together with reference sequences of all representative Fusobacterium members to gain more insight if this is also true for this type of material. This clustering analysis showed that all fusobacteria of the CRC resection material clustered into two distinct groups and every ASV fell into the same group as F. nucleatum and other closely related (sub)species that were previously identified by culturing of the saliva samples (Figure 4a). As shown in Figure 4b, at least one ASV annotated to this genus could be found in all positive patients (i.e., in all patients, except for Patients 12 and 15) and this could even go up to five different ASVs present in two of these patients (i.e., Patients 10 and 11). Most patients carried ASV275, which could be identified in five different patients. ASV numbers 289, 68, 148, and 183 had the highest relative abundance in individual patients. In addition, ASV numbers 289, 68, and 275 had the highest total relative abundance in this cohort.
3.4 All Fusobacterium-Positive CRC Patients Had PCR-Positive Saliva Samples, Possibly Linking Oral and CRC-Associated Fusobacterium
The presence of the genomic content of Fusobacterium, despite the negative culture results in the CRC resection material, led to a reanalysis of the saliva samples. Unfortunately, the obtained DNA was not suitable for 16S rRNA profiling; however, it could still be used for marker gene PCR. The gel electrophoresis (Figure S1) of a genus-specific marker showed that the saliva samples of all patients, except for Patient 15, contained DNA of Fusobacterium, which was also in line with the culture results of these samples.
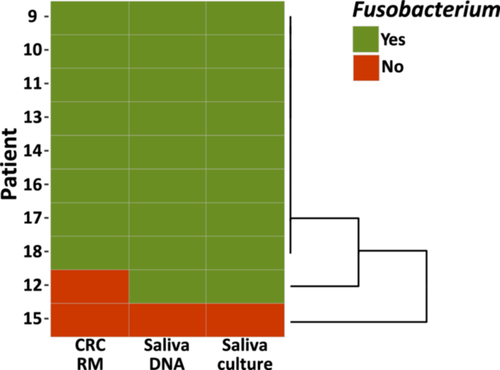
Comparison of the presence of Fusobacterium genomic content between patient-matched saliva samples and CRC resection material (Figure 5) revealed that this pilot group of patients clustered into three subgroups. Eight out of ten patients carried these bacteria both in their oral cavity and in their tumoral site. One patient carried Fusobacterium in the oral cavity, but the presence of this genus was not confirmed in the CRC resection material. Finally, one patient was not a carrier of this genus either in the oral cavity or in the tumor site.
4 Discussion
Culturing methods and genomic analysis were carried out in this study, which showed that Fusobacterium was present in both patient oral samples and CRC resection material. On the basis of saliva and CRC resection material collected from ten CRC patients, Fusobacterium could be identified with culturing in the saliva samples of nine patients, but no Fusobacterium could be isolated from the CRC resection material. On the other hand, 16S rRNA gene profiling of the resection material indicated that eight CRC patients were indeed positive for Fusobacterium; all eight of these patients carried Fusobacterium also in their saliva.
These findings are in accordance with previous studies, that Fusobacterium is prevalent in CRC tumor tissue; however, the origin of these fusobacteria is still unclear. Early evidence suggests that these bacteria might originate from the oral cavity (Castellarin et al. 2012; Kostic et al. 2012; Richardson et al. 2020). Still, more evidence is needed to conclude that CRC-associated fusobacteria indeed have an oral origin. In this study, Fusobacterium was identified in CRC resection material of several patients, along with other pathogenic and opportunistic genera, such as Campylobacter, Burkholderia, and Serratia. Yet, Fusobacterium has already been shown to be involved in a multitude of steps in CRC development and progression (Yu, Kim, and Park 2020; Yang et al. 2017; Rubinstein et al. 2019; Kostic et al. 2013; Gur et al. 2019; Casasanta et al. 2020; Chen et al. 2020; Yu et al. 2017).
To investigate the possible oral origin of fusobacteria in CRC tumor tissue, oral samples were also collected herein. First, different oral sampling methods were studied in a small group of volunteers. In general, there are various oral sampling methods available with relevant differences among each other and the method should be chosen according to the design and aim of a specific research question (Lu et al. 2022). Herein, it was decided to compare GCF with saliva samples as the main aim was to collect Fusobacterium. It has been shown previously that supragingival plaque samples, which would have been a third option, are often more heterogeneous in their constitution compared to subgingival ones, whereas subgingival plaque samples rather consistently harbor Fusobacterium (Zijnge et al. 2010). The present analysis indicated that the culturable species diversity was comparable between saliva and GCF samples. This is in line with previous results from the literature, showing that bacterial compositions with these two sampling methods are comparable (Haririan et al. 2014). However, the two methods differ in their applicability, as GCF sampling is more prone to contamination and thus less suitable for collection in a hospital setting. Taking all this into consideration, the saliva sampling method was used in the cohort of CRC patients. Initially, the objective was to culture and isolate Fusobacterium from oral samples and CRC resection material, after which whole-genome sequencing would be performed on all isolated strains. The oral samples were almost all culture-positive for Fusobacterium, as expected, as Fusobacterium is one of the most prominent oral microbes (Socransky et al. 1998; Motoc et al. 2023). The presence of Fusobacterium was also confirmed with genus-specific marker gene PCR and mirrored the culturing results, showing that a given patient either carried or did not carry this genus. This gave the confidence that the protocol for culture-based screening in this study was appropriate for this type of sample, as the bacterial DNA load corresponded to the presence of viable bacteria. Viable and multiplying bacteria are essential for culture-based screening, which is both the biggest advantage and disadvantage. The advantage is that only bacteria that will grow are detected, and pure colonies can be used subsequently to obtain high-resolution genome sequences. At the same time, the biggest drawback is that bacteria might lose viability before sampling, as live microbial cell loads may fluctuate in orders of magnitude across participants or time points (Marotz et al. 2021) or during every step of the protocol, or they may require other specific growth conditions than those implemented. This drawback was found with the CRC resection material, where no fusobacteria could be isolated from the patient-matched samples using our protocol. Previously, however, we had successfully isolated Fusobacterium during optimization with CRC resection material of four patients, of which one was culture-positive (data not shown). Isolation of these bacteria from this material was already previously described to be difficult, ascribed to the pre-procedural antibiotics that patients receive (Abed et al. 2020). For this reason, antibiotic usage in the 3 preceding months was recorded in our cohort; not all patients had received antibiotics but were still culture-negative for Fusobacterium. To tackle the drawbacks of a culture-based approach, DNA was isolated to perform 16S rRNA amplicon sequencing. Interestingly, 16S rRNA profiling of the resection material revealed that almost all patients who carried oral fusobacteria also had CRC-associated bacteria of this genus, even though some patients received antibiotics within the 3 preceding months, which was previously suggested to reduce the presence of Fusobacterium. However, the resolution of 16S rRNA profiling depends on the amplicon size and, in general, the bacteria could only be identified with confidence to the genus level due to the short amplicon size of 407 base pairs after trimming. Still, clustering of the fusobacterial ASVs after alignment with reference species and with a 95% cut-off value after bootstrapping suggested that all CRC-associated fusobacteria belong to the cluster of F. nucleatum and closely related species, which could be cultured from the oral samples as well. Unfortunately, the quality of the isolated salivary DNA was too poor to perform 16S rRNA gene profiling, possibly due to the relatively low bacterial biomass compared to host DNA and the fact that no bacterial DNA enrichment protocol was implemented, which was previously described to improve sequence quality (Marotz et al. 2021, 2018).
Altogether, these results underline an interesting link between oral and CRC-associated fusobacteria. However, more in-depth analysis is needed to conclude that specific strains are indeed shared between the two locations. In addition, strain-specific genes that are enriched in CRC-associated strains could be analyzed, as previous reports indicated that there is a significant difference in gene composition, for instance, regarding virulence factors or antibiotic resistance (Ma et al. 2023). In particular, the latter is of interest, because antibiotic treatment of patients might ameliorate the clinical outcome by reduction of Fusobacterium load, CRC cell proliferation, and overall tumor growth as shown in mice (Bullman et al. 2017). This in-depth analysis can be carried out with high-resolution metagenomic sequencing in combination with depletion of host DNA to increase the resolution of bacterial reads (Marotz et al. 2018). Finally, from a clinical point of view, it will be interesting to assess in future studies with a larger sample size whether patient-related parameters (e.g., smoking status, intake of antibiotics, comorbidities, etc.), the oral health status per se (e.g., periodontally healthy vs. diseased, severity and extent of periodontal disease, etc.), and/or the oral sampling method (e.g., subgingival vs. supragingival vs. saliva) have an impact on the colonization and/or the detection rate of oral fusobacteria at the CRC site.
5 Conclusion
On the basis of culturing methods and genomic analysis performed in this study, it was shown that Fusobacterium was present in both oral samples and CRC resection material; nevertheless, genomic analysis seems to be more appropriate, as culturing failed to detect any Fusobacterium in the CRC resection material. Overall, the positive pilot results provide us with the confidence to move forward to a bigger cohort to overcome the significant limitations of a small sample group size and perform shotgun metagenomic sequencing on patient-matched oral and CRC samples to map the associated microbiota in high resolution. This will provide the tool to identify whether specific oral strains of Fusobacterium, among others, are indeed associated with CRC and whether particular characteristics are enriched in these tumors.
Author Contributions
Niels Plomp: analysis, interpretation, manuscript drafting. Kristina Bertl: idea, interpretation, sampling, manuscript editing. Marie-Louise Lydrup: sampling, manuscript editing. Klas Sjöberg: design, interpretation, manuscript editing. Hermie J.M. Harmsen: design, interpretation, manuscript drafting, funding. Andreas Stavropoulos: idea, interpretation, sampling, funding, manuscript editing.
Acknowledgments
The present trial was financially supported by the Crafoord Foundation, the IADR-Philips Oral Healthcare Young Investigator Research Grant, and the OFRS (Odontologisk Forskning i Region Skåne). N.P. was supported by a grant from the Graduate School of Medical Sciences of the University of Groningen, The Netherlands.
Ethics Statement
The protocol was approved by the regional ethical review board (Dnr. 2016/469).
Consent
All participants signed oral and written consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




