Clinically Important Endpoints in Individuals With Leukodystrophy: A Multisite Study
ABSTRACT
Importance
Leukodystrophies are a diverse group of rare disorders that disrupt central myelination. These disorders present with a broad spectrum of neurological severity and are associated with a range of potential secondary complications, such as scoliosis and failure of independent feeding.
Objective
We explore real-world data of leukodystrophy complications to inform future evidence-based care guidelines across these rare diseases.
Design
In a cross-sectional observational study, we use a leukodystrophy-specific research consortium and the availability of electronic health records (EHR) to capture a cross-section of real-world data.
Setting
Study participants were identified using EHR data from five hospital systems with established expertise in leukodystrophies.
Participants
Principal investigators or genetic counselors confirmed leukodystrophy diagnoses in all participants.
Exposures
Time-to-event measures were collected, including orthopedic complications (scoliosis, hip subluxation/dislocation), loss of ambulation, artificial ventilation, gastrostomy tube placement, and urinary tract infections (UTIs). Maximum motor milestones, including gain of ambulation by 2 years of age, were captured to stratify cohorts by neurological severity.
Main Outcome and Measure
A primary outcome was not prespecified, as this was an observational study.
Results
In total, 1203 participants were identified across 42 leukodystrophies (age range of 9 days to 89 years at last encounter). The most common event was feeding tube placement, and the median time to any first complication varied between disorders (Fleming–Harrington weighted log-rank test). The specific diagnosis correlated with maximum gross motor milestone attainment (chi-square test of independence). When all disorders were stratified by maximum motor milestone attainment (not specific diagnosis), the median time to adverse events was significantly associated with function (Fleming–Harrington weighted log-rank test).
Conclusions
This cross-sectional, retrospective observational study demonstrated that key medical events could be identified across institutions using EHR capture. Rates of health events and attainment of maximum motor milestones varied by specific leukodystrophy type. However, when the overall cohort was stratified by severity of motor impairment rather than individual diagnoses, the frequency of health events was associated with motor function. Our findings suggest utility for development of comprehensive care models for leukodystrophies as stratified by motor function and demonstrate the utility of integrated EHR review for real-world data analyses.
1 Introduction
Leukodystrophies are a diverse group of heritable disorders that affect the myelination of the central nervous system. Universally, leukodystrophies result in a profound impact on health-related quality of life and overall functionality [1-3]. Many leukodystrophies are defined by their impact on mobility [4-7], impairment of swallowing abilities [8], increased tone with secondary orthopedic complications such as hip dislocation [8], urinary tract infections (UTIs) [9], and respiratory failure [10, 11]. As a group, the leukodystrophies have a high likelihood of these complications, although there are limited data on the prevalence of these complications and their impact on morbidity and mortality [12-14].
Two leukodystrophies are now included on the Recommended Uniform Screening Panel: adrenoleukodystrophy (ALD) in 2016 [15] and Krabbe disease in 2024 [16]. As such, there are also now emerging cohorts of pre- and postsymptomatic individuals. This expanding spectrum of disease has led to challenges with anticipatory guidance, given the lack of evidence behind potential complications in large cohorts. This is highlighted in the case of ALD, which has discrete neurological subtypes based on imaging and age of onset: childhood-onset cerebral, adult-onset cerebral, adult-onset adrenomyeloneuropathy (AMN), and a sizeable presymptomatic cohort identified through newborn screening [17].
This study leveraged a research consortium and electronic health record (EHR) systems to identify a large cohort of individuals with leukodystrophies. The objective was to define the variability of key health events across a spectrum of disorders. Given the limited number of patients with leukodystrophy at a single institution, cross-institutional EHR sharing has the potential to build comprehensive data sets, enhancing insights into disease progression and variability. This broader data pool supports more effective clinical trials and helps clinicians make evidence-based decisions, ultimately leading to better patient outcomes.
2 Methods
This is a cross-sectional observational study. See Supplemental Methods for additional details on data collection and event selection.
2.1 Participant Inclusion
Affected individuals were identified based on an exempt protocol (IRB# 20-018050) approved at multiple sites, including the Children's Hospital of Philadelphia (CHOP), Children's Healthcare of Atlanta (CHOA), Kennedy Krieger Institute (KKI), Massachusetts General Hospital (MGH), and Stanford University. All sites participate in the Rare Disease Clinical Research Network (RDCRN)– funded GLIA-CTN and use a common EHR (Epic Systems Inc.). Data were extracted from May 2021 to November 2023 across the entire available data set from EHR implementation at that site until the completion of data extraction (Supporting Information S1: Table 1).
EHR data were extracted at each site to identify study participants using an automated search for keywords in narrative documentation (e.g., mentions of relevant gene variants) and by ICD9/ICD10 codes (Supporting Information S1: Table 2). The diagnosis was manually verified by the genetic counselors of the GLIA-CTN genomics core or by principal investigators by review of source documentation of biochemical and/or molecular testing. Participants without a confirmed diagnosis or with potentially confounding co-diagnoses were excluded (Supporting Information S1: Table 3). Because ALD has known age-based subtypes with different neurological trajectories, individuals with ALD were further divided into presymptomatic individuals identified by newborn screening (those born after 03/01/2016), individuals identified with childhood-onset ALD, including cerebral ALD (those with ALD under 18 years of age with or without a clinical event), and adults with ALD, including adult cerebral ALD and AMN (those 18+ years of age with or without a clinical event).
2.2 Data Collection
Charts in the EHR were reviewed manually to identify the occurrence and age of key time-to-events (TTEs) using standardized search terms. Both structured (laboratory reports, surgical procedures) and unstructured (radiology reports and progress notes) data sources were queried. Each TTE outcome was categorized as (1) Noted, (2) Not Noted, and (3) Unknown/Not Reported at the time of the last medical records captured. The number of treated individuals by diagnosis and type of targeted treatment (e.g., bone marrow transplant or gene therapy) was recorded (Supporting Information S1: Table 9), and data were compared between stratified treatment subcohorts as well as by censoring individuals at the time of treatment.
2.3 Statistical Analysis (see supplemental)
Diagnosis strata with subgroup sizes with at least 20 participants were included for diagnosis-specific analyses. Descriptive analyses include the generation of mean, standard deviation (SD), median, interquartile range (IQR), and range for continuous variables (e.g., ages at TTE outcomes) and frequency and percentage for categorical variables. Demographic distributions were compared between study sites using Kruskal–Wallis testing for continuous variables and chi-square testing for categorical variables. Conditional event probabilities were described for scoliosis surgery by event status, hip surgery by event status, feeding tube placement by ambulation status (none, 5 years of age and earlier, and after 5 years), loss of ambulation by feeding tube placement status (2 years of age and earlier or after 2 years), and UTI by loss of ambulation status (Supporting Information S1: Table 7). Kaplan–Meier curves and Fleming–Harrington weighted log-rank tests were used to determine differences in median time to the first clinical event between strata. Due to their large population sizes and broad distribution of motor function, Cox proportional hazards regression was used to evaluate the association between max motor milestone attainment and diagnosis with the hazard of initial clinical events in Aicardi-Goutières syndrome (AGS, n = 117) and TUBB4A-related leukodystrophy (TUBB4A-LD, n = 45). Proportional hazards assumptions were evaluated graphically with log-log plots and global goodness of fit testing. All testing used an alpha level of 0.05, and 95% confidence intervals were calculated. Where confidence interval limits were not estimable due to decreasing sample size from censoring, limits were denoted as “NE.” Sensitivity analysis for treatment effect was performed by stratifying the cohort by diagnoses with substantial treated subcohorts (AGS, Allan-Herndon-Dudley syndrome [AHDS, ALD, metachromatic leukodystrophy [MLD]) and max motor milestone group and comparing TTE between treated subcohorts as well as censoring individuals at the time of treatment.
3 Results
3.1 Participants
A total of 1203 patients were identified as having a diagnosis of leukodystrophy. Distribution differences were seen across the site cohorts by diagnosis, age at the most recent encounter, and birth year (Table 1; chi-square test, all p < 0.001). Data on more than 40 diagnoses were captured through EHR extraction across a range of follow-up times (Figure 1A). There were also differences in the range of years captured depending on when the EHR was introduced at each site. Most events were documented in 2010 or later (83.31%) (Table 1). The median age at final follow-up for all disorders was 11.4 (IQR = 17.3) years and varied by disorder based on typical ages at presentation (Table 1; Figure 1A).
| CHOP | CHOA | MGH | KKI | Stanford | p | Overall | |
|---|---|---|---|---|---|---|---|
| (N = 535, 44.5%) | (N = 203, 16.9%) | (N = 266, 22.1%) | (N = 116, 9.6%) | (N = 83, 6.9%) | (N = 1203) | ||
| Diagnosis | |||||||
| AGS | 107 (20.0%) | 6 (3.0%) | 4 (1.5%) | 6 (5.2%) | 0 (0%) | < 0.001 | 123 (10.2%) |
| AHDS | 16 (3.0%) | 5 (2.5%) | 0 (0%) | 2 (1.7%) | 0 (0%) | 23 (1.9%) | |
| AxD | 87 (16.3%) | 4 (2.0%) | 9 (3.4%) | 3 (2.6%) | 2 (2.4%) | 105 (8.7%) | |
| Canavan | 13 (2.4%) | 2 (1.0%) | 7 (2.6%) | 1 (0.9%) | 0 (0%) | 23 (1.9%) | |
| Krabbe | 15 (2.8%) | 14 (6.9%) | 6 (2.3%) | 1 (0.9%) | 4 (4.8%) | 40 (3.3%) | |
| MLD | 41 (7.6%) | 21 (10.3%) | 0 (0%) | 5 (4.3%) | 12 (14.5%) | 79 (6.6%) | |
| Other | 85 (15.9%) | 87 (42.9%) | 22 (8.3%) | 9 (7.8%) | 0 (0%) | 203 (16.9%) | |
| PBD | 15 (2.8%) | 19 (9.4%) | 0 (0%) | 4 (3.4%) | 5 (6.0%) | 43 (3.6%) | |
| PMD | 22 (4.1%) | 13 (6.4%) | 0 (0%) | 7 (6.0%) | 3 (3.6%) | 45 (3.7%) | |
| POLR3-LD | 22 (4.1%) | 4 (2.0%) | 1 (0.4%) | 0 (0%) | 2 (2.4%) | 29 (2.4%) | |
| TUBB4A-LD | 44 (8.2%) | 1 (0.5%) | 0 (0%) | 4 (3.4%) | 0 (0%) | 49 (4.1%) | |
| VWM | 8 (1.5%) | 4 (2.0%) | 8 (3.0%) | 0 (0%) | 0 (0%) | 20 (1.7%) | |
| ALD | 60 (11.2%) | 23 (11.3%) | 209 (78.6%) | 74 (63.8%) | 55 (66.3%) | 421 (35.0%) | |
| Birth year category | |||||||
| > 2010 | 301 (56.3%) | 111 (54.7%) | 47 (17.7%) | 31 (26.7%) | 52 (62.7%) | < 0.001 | 542 (45.1%) |
| 2000–2010 | 163 (30.5%) | 70 (34.5%) | 63 (23.7%) | 15 (12.9%) | 14 (16.9%) | 325 (27.0%) | |
| < 2000 | 71 (13.3%) | 22 (10.8%) | 156 (58.6%) | 70 (60.3%) | 17 (20.5%) | 336 (27.9%) | |
| Age at recent encounter (years) | |||||||
| Mean (SD) | 12.3 (10.2) | 8.4 (6.22) | 31.0 (20.9) | 32.6 (22.0) | 13.7 (13.9) | < 0.001 | 17.9 (17.3) |
| Median (IQR) | 9.1 (11.8) | 7.3 (9.30) | 28.4 (34.8) | 30.2 (39.1) | 9.2 (14.7) | 11.4 (17.3) | |
| [Min, max] | [1.1, 65.4] | [0.03, 25.8] | [0.2, 89.3] | [2.4, 79.5] | [0.30, 64.7] | [0.03, 89.3] | |
| [Q1, Q3] | [5.1, 16.9] | [3.2, 12.5] | [12.5, 47.3] | [11.6, 50.7] | [4.1, 18.8] | [5.6, 23.0] | |
- Abbreviations: AxD, Alexander disease; PBD, peroxisomal biogenesis disorders; PMD, Pelizaeus-Merzbacher disease; POLR3-LD, POLR3-related leukodystrophy; VWM, vanishing white matter disease.
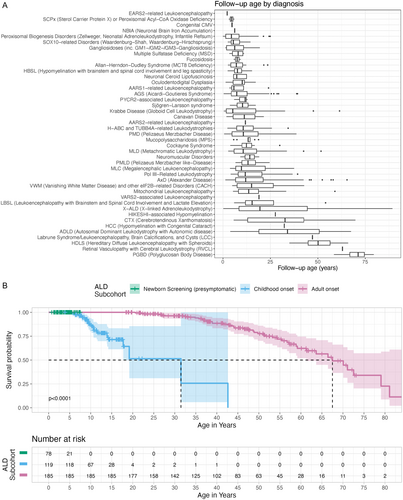
The largest cohort was X-linked adrenoleukodystrophy (ALD; n = 421, 35.0%; Supporting Information S1: Table 2A), followed by AGS (n = 123) and Alexander disease (AxD; n = 105). Within ALD, three subcohorts were defined: presymptomatic individuals (n = 89, 21.1%), childhood-onset cerebral ALD (n = 137, 32.5%), and adult ALD, including adult cerebral ALD and AMN (n = 195, 46.3%).
3.2 Diagnosis and Distribution of Significant Clinical Events
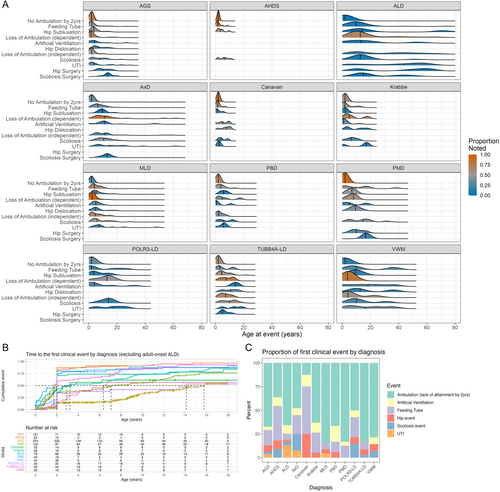
Next, the onset of key medical events was collected to capture the burden of systemic disease. These events included feeding tube placement, orthopedic complications (e.g., scoliosis, scoliosis surgery, hip subluxation or dislocation, and hip surgery) and the use of artificial ventilation (e.g., continuous positive airway pressure [CPAP], bilevel positive airway pressure [BiPAP], or endotracheal tube placement), and UTIs. The median age at first clinical complication varied by disorder (Fleming–Harrington log-rank test, p < 0.001) (Figure 2A,B). Ambulation events and feeding tube placement were the most common first clinical events, while artificial ventilation, orthopedic complications, or UTI generally occurred later (Figure 2A,C; Supporting Information S1: Table 10). Notably, AxD had a large proportion of scoliosis events (12, 17.4%) before other complications (Figure 2C; Supporting Information S1: Table 10).
3.3 Gain and Loss of Gross Motor Skills
The maximum motor milestone attained was associated with specific diagnosis (chi-square test for independence, p < 0.001; Figure 3). The majority of individuals within ALD (n = 388, 96.0%), vanishing white matter disease (VWM) (n = 17, 85.0%), POLR3-related leukodystrophy (POLR3-LD) (n = 20, 69.0%), metachromatic leukodystrophy (MLD) (n = 52, 76.5%), and AxD (n = 80, 80.0%) attained independent ambulation. Individuals with Canavan disease primarily attained only head control (n = 8, 66.7%). Pelizaeus-Merzbacher disease (PMD) and Allan-Herndon-Dudley syndrome (AHDS) cohorts demonstrated a similar distribution of gross motor function when compared with each other (chi-square test for independence, p = 0.97), with the majority of individuals attaining sitting (n = 12, 35.3% and n = 6, 35.3%, respectively) and dependent ambulation (n = 12, 35.3% and n = 5, 29.4%, respectively). AGS, TUBB4A-LD, Krabbe disease, and peroxisomal biogenesis disorder (PBD) also had similar distributions of motor function with a broad distribution of maximum attainment across the gross motor milestones (chi-square test for independence, p = 0.57). Overall, the disorders with a greater proportion of ambulatory individuals had a later first complication (Figure 2B; Supporting Information S1: Table 8).
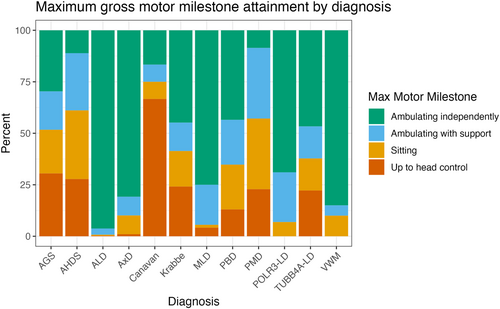
The age at loss of ambulation was also captured (Supporting Information S1: Figure 3). The median time to loss of ambulation in the ambulatory participants with TUBB4A-LD was 8.0 years (95% CI, 7.0-NE), with MLD was 10.4 years (95% CI, 7.3–20.1), with AGS was 11.0 years (95% CI, 3.0-NE), with VWM was 23.1 years (95% CI, 6.4-NE), with POLR3-LD was 32.1 years (95% CI, 32.1, NE), with AxD was 44.7 years (95% CI, 31.0-NE), and with ALD was 66.4 years (95% CI, 58.9-NE). In PMD, AHDS, and Canavan disease, only two participants were ambulatory and did not lose ambulation during the follow-up period. Ambulatory participants with Krabbe and PBD did not reach the median time-to-loss of ambulation.
Notably, the ALD cohort includes children identified by newborn screening who are asymptomatic, children affected by cerebral ALD with and without treatment, and adult AMN. Each subtype was evaluated individually as well. The median time to loss of ambulation for the childhood ALD subcohort occurred at 42.6 years (95% CI, NE-NE) and 70.1 years (95% CI, 62.7-NE) in adult-onset ALD censored at the time of treatment (Figure 1B).
3.4 Morbidity of Adverse Clinical Events by Motor Skills
3.4.1 Feeding Tube Placement
Across disorders, the time to first feeding tube placement significantly differed by maximum motor milestone attainment (Fleming-Harrington weighted log-rank, p < 0.001). The median time to feeding tube placement was earliest in the least mobile groups at 2.3 years (up to head control: 95% CI, 1.9–6.2) and 7.1 years (up to sitting: 95% CI, 4.3–12.0) (Table 2; Figure 4).
| Strata | N | N events | Median (years) | 0.95 LCL | 0.95 UCL | p |
|---|---|---|---|---|---|---|
| Feeding tube placement | ||||||
| Ambulating independently | 678 | 128 | 74.6 | 74.6 | NE | < 0.001 |
| Ambulating dependently | 116 | 49 | 24.3 | 11.5 | NE | |
| Sitting | 96 | 54 | 7.1 | 4.3 | 12 | |
| Up to head control | 89 | 62 | 2.3 | 1.9 | 6.2 | |
| Artificial ventilation | ||||||
| Ambulating independently | 694 | 79 | NE | 72.3 | NE | < 0.001 |
| Ambulating dependently | 119 | 25 | 44.7 | 26.6 | NE | |
| Sitting | 92 | 18 | NE | NE | NE | |
| Up to head control | 88 | 26 | NE | 11.7 | NE | |
| Urinary tract infection | ||||||
| Ambulating independently | 681 | 103 | 60.6 | 58.1 | 64.4 | < 0.001 |
| Ambulating dependently | 110 | 21 | 38.9 | 25.8 | NE | |
| Sitting | 84 | 13 | NE | 18.4 | NE | |
| Up to head control | 84 | 22 | 23.8 | 23.8 | NE | |
| Scoliosis | ||||||
| Ambulating independently | 686 | 145 | 67.5 | 61.4 | NE | < 0.001 |
| Ambulating dependently | 120 | 58 | 14.6 | 11.8 | 20.4 | |
| Sitting | 90 | 29 | 12.7 | 8.8 | NE | |
| Up to head control | 86 | 43 | 11.5 | 7.2 | 14.2 | |
| Scoliosis surgery | ||||||
| Ambulating independently | 663 | 20 | NE | NE | NE | < 0.001 |
| Ambulating dependently | 109 | 13 | NE | NE | NE | |
| Sitting | 85 | 6 | NE | 14.7 | NE | |
| Up to head control | 86 | 7 | 23.3 | 19.6 | NE | |
| Hip subluxation | ||||||
| Ambulating independently | 656 | 57 | NE | NE | NE | < 0.001 |
| Ambulating dependently | 111 | 54 | 9.7 | 8.2 | 22.4 | |
| Sitting | 90 | 37 | 9.4 | 6.2 | NE | |
| Up to head control | 87 | 44 | 8.5 | 5.2 | 13.9 | |
| Hip dislocation | ||||||
| Ambulating independently | 655 | 30 | NE | NE | NE | < 0.001 |
| Ambulating dependently | 108 | 23 | NE | 30.3 | NE | |
| Sitting | 88 | 22 | 14.6 | 10.9 | NE | |
| Up to head control | 86 | 34 | 12.7 | 8.5 | 15.4 | |
| Hip event (subluxation or dislocation) | ||||||
| Ambulating independently | 654 | 68 | NE | NE | NE | < 0.001 |
| Ambulating dependently | 109 | 55 | 9.5 | 7.1 | 22.4 | |
| Sitting | 90 | 39 | 7.6 | 6.2 | NE | |
| Up to head control | 87 | 55 | 6.9 | 4.6 | 9.5 | |
| Hip surgery | ||||||
| Ambulating independently | 657 | 17 | NE | NE | NE | < 0.001 |
| Ambulating dependently | 109 | 19 | NE | NE | NE | |
| Sitting | 88 | 9 | 25.5 | 15.8 | NE | |
| Up to head control | 87 | 18 | 21.2 | 17.5 | NE | |
| Mortality | ||||||
| Ambulating independently | 68 | 19 | 89.3 | 73.4 | NE | < 0.001 |
| Ambulating dependently | 45 | 2 | NE | NE | NE | |
| Sitting | 42 | 4 | NE | NE | NE | |
| Up to head control | 35 | 7 | NE | 11.5 | NE | |
- Abbreviations: LCL, lower control limit; NE, not estimable; UCL, upper control limit.
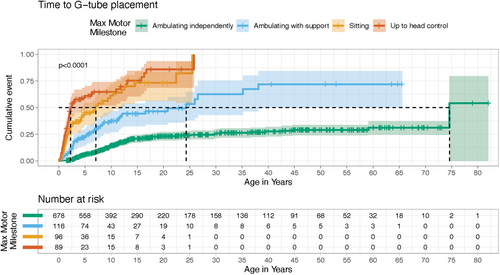
The AGS and TUBB4A-LD cohorts were used in additional analyses because of the cohort size and broad clinical spectrum. Within the AGS and TUBB4A-LD cohorts, the probability of a feeding tube placement was also highest in participants who lost ambulation before 5 years (56%) compared with those who lost ambulation after 5 years (44%) or did not lose ambulation (8%) (Supplemental Results; Supporting Information S1: Table 7). Similarly, participants with a feeding tube placement before 2 years were more likely to be non-ambulatory at 2 years of age (80%) compared with those with a feeding tube placement after 2 years (33%, 47% difference) or without a feeding tube placed (16%, 64% difference) (Supporting Information S1: Table 7).
3.4.2 Orthopedic Complications
Scoliosis occurred in 315 patients (28.4%; Supporting Information S1: Figure 1C), although surgery was less common (55, 5.17%) (Supporting Information S1: Figure 1C). Study participants who attained ambulation had a later median time to scoliosis (60.6 years; 95% CI, 58.1–64.4), whereas the non-ambulatory groups were similar: 11.5 years (95% CI, 7.2–14.2) in up to head control, 12.7 years (95% CI, 8.8-NE) in sitting, and 14.6 years (95% CI, 11.8–20.4) in dependent ambulation (Fleming–Harrington log-rank test, p < 0.001; Table 2; Supporting Information S1: Figure 5B). For AGS and TUBB4A-LD, the hazard of scoliosis was not significantly associated with diagnosis but was significantly associated with maximum motor milestones (likelihood ratio tests, p = 0.60 and p = 0.02, respectively).
Most notable hip-related complications were subluxation (214, 20.1%) and dislocation (136, 12.8%). Surgical intervention of the hips was rare in the overall cohort (75, 7.08%) (Supporting Information S1: Figure 1B). For patients with documented hip events, the probability of surgery during the follow-up period was 28%; seven participants had known surgeries with no documented prior hip event (1%) (Supporting Information S1: Table 7). Lack of motor milestone attainment was significantly associated with the hazard of orthopedic outcomes (Fleming-Harrington log-rank test, all p < 0.001; Table 2). Median time to first hip event (subluxation or dislocation) was reached for only non-ambulatory maximum motor milestone groups: 6.9 years up to head control (95% CI, 4.6–9.5), 7.6 years sitting (95% CI, 6.2-NE), and 9.5 years supported ambulation (95% CI, 7.1-22.4) (Table 2; Supporting Information S1: Figure 5F).
In the AGS and TUBB4A-LD subgroup, the hazard of both hip subluxation and hip dislocation was significantly associated with maximum motor milestone attainment (likelihood ratio tests, p < 0.001 and p = 0.003, respectively; Supporting Information S1: Table 6) but was not significantly associated with diagnosis (likelihood ratio tests, p = 0.18 and p = 0.74, respectively).
3.4.3 Urinary Tract Infection (UTI)
UTIs were documented in 180 patients (16.6%). In all diagnoses, median time to UTI differed by maximum motor milestone attainment group (Fleming–Harrington weighted log-rank test, p < 0.0001). The median time to UTI for participants who attained up to head control, dependent ambulation, and independent ambulation was 23.8 years (95% CI, 23.8-NE), 38.9 years (95% CI, 25.8-NE), and 60.6 years (95% CI, 58.1–64.4), respectively (Table 2; Supporting Information S1: Figure 5A).
In AGS and TUBB4A-LD, UTI was associated with diagnosis but not with maximum motor milestone attainment (likelihood ratio test, p = 0.002 and p = 0.50, respectively). The hazard of UTI for participants with TUBB4A-LD was significantly lower than in participants with AGS (hazard ratio, 0.2; 95% CI, 0.1–0.7; p = 0.01; Supporting Information S1: Table 6), which may represent their treatment with immunosuppressive agents that impact the risk of UTI.
3.4.4 Artificial Ventilation
Events related to artificial ventilation were documented in 189 patients (16.8%). In all diagnoses, median time to artificial ventilation was different between maximum motor milestone groups (Table 2; Fleming–Harrington weighted log-rank test, p < 0.001).
3.4.5 Mortality
In the cohort, 32 patients (5.8%) died during the follow-up period. The median survival time was reached during the observation period only for those who attained independent ambulation (median, 89.3 years; 95% CI, 73.4-NE; Table 2), which is similar to the general population (76.4 years) [18]. The other maximum motor milestone attainment strata did not reach median survival. Although highly variable, mortality by 10 years of age among the patients who only achieved head control was 23% (95% CI, 1%–40%), for those who attained up to sitting was 29% (95% CI, 0%–50%), for those who attained dependent ambulation was 3% (95% CI, 0%–9%), and for those who attained independent ambulation was 2% (95% CI, 0%–3%).
3.4.6 Effect of Disease-Modifying Therapies
Of our total cohort, 998 individuals were not treated, while 205 individuals received a disease-modifying therapy, including 50% of children with AGS (61/123), 43% of AHDS, 29% of MLD (23/79), 19% of ALD (79/421), 18% of Krabbe (7/40), and 17% of Canavan (4/23) (Supporting Information S1: Table 9). While in some cases, therapies are approved by the Food and Drug Administration or are standard of care, in many cases, these were participants treated in clinical trials. Only in AHDS and AGS, for time to hip subluxation (Supporting Information S1: Table 12, p = 0.049 and 0.040) were time-to-event measures significantly different across any of the treatment groups, in favor of untreated individuals, though their ultimate time to hip surgery was unchanged, suggesting an effect on earlier detection of hip abnormalities in patients evaluated in a clinical trial. Similarly, children in treatment whose maximum motor milestone was supported ambulation were more likely to be identified as having hip subluxation earlier (Supporting Information S1: Table 13, p < 0.0001) and more likely to undergo surgery (Supporting Information S1: Table 13, p < 0.0001). Children whose maximum motor milestone was head control were more likely to have a detected UTI (Supporting Information S1: Table 13, p = 0.0004). There was no change in the significance of the results of maximum motor milestone across all outcomes when data were censored at the time of treatment initiation for the 17% of individuals receiving an intervention.
4 Discussion
This study compiles the single largest leukodystrophy cohort, through a collaborative consortium of academic medical centers with review of EHRs. In contrast, current understanding of leukodystrophies is primarily based on small cross-sectional studies from single institutions, complicated by participation and reporting biases. In this study, we defined characteristic disease-specific patterns of complications. By stratifying diagnoses based on the maximum motor milestone attained, we identified overall motor severity as an important driver of potential complications. This finding suggests potential generalizability of clinical monitoring and management across leukodystrophies [10]. Similarly, mortality was highest in individuals with the lowest attainment of motor milestones. While some children in this study received disease-modifying therapy clinically or as part of a clinical trial, outcomes studied in our work (need for feeding tube, maximum motor milestone) are unrelated to the outcomes of clinical trials both temporally and mechanistically. Our data suggest earlier detection of leukodystrophy-related morbidity in children followed during treatment protocols, presumably due to a bias created by increased monitoring. Our findings support improved anticipatory care based on motor attainment and prior health events.
Extraction of clinically relevant data from EHRs allows for the creation of large data sets within rare disease populations but has faced challenges in rigor. In this study, we used parallel manual and automated approaches to improve rigor and reproducibility. One of the most important challenges was related to population identification due to a lack of disease-specific ICD-10 codes. As such, participants were identified by an automated search for keywords in narrative documentation (e.g., mentions of relevant gene variants), supplemented by ICD-9/ICD-10 codes, and then manually verified, resulting in a genetically confirmed cohort of 1203 leukodystrophy patients. This approach decreased the number of false positives, such as when MRI changes were misattributed to a leukodystrophy. This EHR-based approach also has the potential benefit of reducing recruitment bias. The importance of using a multi-institutional structure was highlighted in the cohort differences contributed by each site, both by age and disease.
To additionally reduce the impact of limitations associated with structured EHR data, we used automated and manual approaches to leverage additional information only available in free-text format (e.g., progress notes). We used an automated approach to screen narrative information in the EHR of patients eligible for inclusion in our cohort. At all sites, we supplemented our automated screening process with a manual review process to determine the presence and timing of clinically important outcome events. Future studies could explore additional disease-related features, such as seizures, as well as capture additional findings, such as imaging or laboratory testing results. This study is nevertheless limited by the information available within the EHR, such as the documentation of out-of-hospital deaths.
The occurrence of events may be biased by evolving standards of care as well as regional differences in access. We hypothesize that some events, such as artificial ventilation, were not uniformly offered to all individuals as part of end-of-life care [10]. As more leukodystrophy populations begin to receive disease-modifying treatments, the events within the disease's natural history will evolve as ages and types of medical events occurring in treated patients may change. Future studies, inclusive of additional sites, have the potential to analyze the impact of year and region on health interventions and outcomes. Finally, these data only represent US-based care approaches, and frequency and timing of health outcomes may be different in international cohorts.
In summary, we show that a combination of automated and manual review can identify and collect standardized information from EHR systems for a large cohort of patients with rare neurogenetic conditions (leukodystrophies) across sites. By stratifying diagnoses based on the maximum motor milestone attained, we identified overall motor severity as an important driver of potential complications. A comprehensive understanding of the burden of disease associated with the leukodystrophies has important potential benefits. This can be used to build evidence-based care guidelines, inform anticipatory care with newly diagnosed families, and evaluate potential side effects versus disease-related complications in the context of clinical trials. This study can also help to inform our understanding of the burden of care throughout the lifespan for individuals affected by leukodystrophies. Our findings suggest the potential to establish standards for care and for outcomes, which can be used to reduce disparities currently observed for children with leukodystrophies [19, 20]. The next steps based on this study should involve the development of care guidelines and inform clinical trial design.
Author Contributions
Emma R. Kotes, Jacob McCann, Jeilo Gauna, Dandre Amos, Jordan Goodman, Seungil Lee, and Nicole Page: data collection. Sarah Woidill: data curation, formal analysis. Russell D'Aiello, Amina Khan, and Mark Ramos: data collection, data curation. Francesco Gavazzi, Stephanie Keller, Keith Van Haren, Ali Fatemi, Florian Eichler, Ashley Hackett, Amena Smith Fine, Amanda Nagy, Mariko Bennett, Joshua Bonkowsky, Jamie Fraser, Lisa Emrick, and Amy Waldman: writing – review and editing, revision. Laura Adang: original draft, writing – review and editing, revision. Johanna Schmidt, Amy Pizzino, and Kayla Muirhead: data review, subject identification. Justine Shults: statistical analyses, statistical analysis plan. Robert Grundmeier and Adeline Vanderver: conceptualization, design, writing – original draft, writing – review and editing.
Acknowledgments
We thank Meena Verma for her abstraction efforts with the Children's Healthcare of Atlanta group and Natalie Grant for her efforts with the Massachusetts General Hospital group. The study was funded by the National Institutes of Health (NIH) grant U54NS115052.
Conflicts of Interest
All authors of the manuscript were funded by the U54NS115052 grant. E.R. Kotes reports no disclosures relevant to the manuscript. S. Woidill reports no disclosures relevant to the manuscript. R. D'Aiello receives support from Affinia, Biogen, Ionis, Orchard, and Sana. A. Khan reports no disclosures relevant to the manuscript. J. McCann reports no disclosures relevant to the manuscript. M. Ramos reports no disclosures relevant to the manuscript. F. Gavazzi reports no disclosures relevant to the manuscript. S. Keller reports no disclosures relevant to the manuscript. K. Van Haren is an editorial board member of Annals of the Child Neurology Society. A. Fatemi receives institutional support from the NICHD (P50HD103538). F. Eichler reports no disclosures relevant to the manuscript. A. Hackett reports no disclosures relevant to the manuscript. J. Gauna reports no disclosures relevant to the manuscript. D. Amos reports no disclosures relevant to the manuscript. J. Goodman receives institutional support from the NICHD (P50HD103538). A. Smith Fine receives institutional support from the NICHD (P50HD103538). A. Nagy reports no disclosures relevant to the manuscript. J.L. Bonkowsky writes content for UpToDate, has consulted for Ionis, and is an editorial board member of Annals of the Child Neurology Society. S. Lee reports no disclosures relevant to the manuscript. N. Page reports no disclosures relevant to the manuscript. J. Schmidt reports no disclosures relevant to the manuscript. A. Pizzino reports no disclosures relevant to the manuscript. K. Muirhead reports no disclosures relevant to the manuscript. M. Bennett reports no disclosures relevant to the manuscript. A. Waldman reports no disclosures relevant to the manuscript. L. A. Adang reports no disclosures relevant to the manuscript. J. Shults reports no disclosures relevant to the manuscript. R. Grundmeier reports no disclosures relevant to the manuscript. A. Vanderver reports no disclosures relevant to the manuscript.
Open Research
Data Availability Statement
In addition to study protocols, the statistical analysis plan, the informed consent form, and deidentified individual participant data (including data dictionaries) will be made available upon publication to researchers who provide a methodologically sound proposal to achieve the goals of the approved proposal. Proposals should be submitted to the corresponding author.




