“Ears of the Lynx” Sign on Brain MRI in Siblings With Spastic Paraplegia: A Case Report
ABSTRACT
Background
Hereditary spastic paraplegia (HSP) is a rare, clinically and genetically heterogenous condition that selectively affects the terminal segment of the descending corticospinal tract of the lumbar spine area, causing lower extremity spastic weakness with or without associated complex neurological symptoms. HSP type 11 is the most common form of autosomal recessive HSP and has unique clinical and neuroimaging features.
Methods
We describe the clinical manifestations and imaging features of siblings with childhood-onset autosomal recessive HSP. Genetic testing confirmed compound heterozygous spastic paraplegia gene (SPG) 11 mutations.
Results
The older brother developed poor balance and progressive difficulty with walking starting in childhood. He also experienced poor memory and urinary incontinence. He was born preterm at 30 weeks and was developmentally delayed and cognitivly impaired. His examination revealed length-dependent corticospinal tract signs. Magnetic resonance imaging (MRI) showed thinning of the corpus callosum and periventricular signal changes. His earlier cerebral palsy (CP) diagnosis was based on the history and imaging findings, but rcognition of the “ears of the lynx” MRI sign led to the correct diagnosis of HSP 11 with the Invitae HSP gene panel. The younger sister has similar but milder manifestations and, has the same mutation as her brother.
Interpretation
Although the manifestations of HSP in children often mimic those of CP, the management and progression of HSP differ substantially.
1 Introduction
Hereditary spastic paraplegia (HSP) is a clinically and genetically heterogenous condition that selectively affects the corticospinal tract, among other central nervous system areas, causing lower extremity spastic weakness, with or without other neurological symptoms [1]. Based on Harding's classification, HSP can be classified as either pure HSP or complex HSP [2]. HSP can also be classified as autosomal dominant, autosomal recessive, X-linked, or mitochondrial HSP [3].
More than 80 gene variants have been associated with HSP. These genes have been mapped to almost all chromosomes except chromosomes 5, 20, 21, 22, and Y [3]. HSP was first clearly described in two siblings by the German neurologist Adolph Strumpell in 1880, a phenotype that was later referred to as the pure form of HSP [4]. The first description of the complex form of HSP was reported in 1916 by Philadelphia neurologist John Rhein [5]. Accurate clinical HSP classification was finally developed in the 1980s based on the work of Anita Harding [2]. The first gene responsible for HSP was identified in 1994 and was named spastic paraplegia gene (SPG) 11 [5]. Thereafter, more SPG genes were identified [5, 6]. The prevalence of the different genetic subtypes is unclear. Autosomal dominant forms are more prevalent in North America and North Europe, while autosomal recessive forms are more prevalent in the Middle East and in American Amish groups, likely due to higher consanguinity. Mitochondrial and X-linked forms of HSP are relatively rare compared with the autosomal dominant and recessive forms [7].
The age of onset of HSP varies from infancy to the seventh decade. Its natural history is chronic and slowly progressive [8]. Childhood-onset complex HSPs are often accompanied by central and peripheral nervous system symptoms along with progressive limb spasticity [9]. These symptoms include developmental delay, cerebellar dysfunction, ataxia, seizure, vision and hearing difficulties, and peripheral neuropathy, which complicate the diagnostic process [10]. The differential diagnosis of HSPs is broad and includes acquired myelopathies, inherited metabolic diseases, and other neurodegenerative diseases [3]. In children, HSP can be misdiagnosed as cerebral palsy (CP) [10].
Timely diagnosis of HSP remains a challenge due to its insidious onset, broad range of nonspecific symptoms, and slowly progressive nature. Next-generation genetic sequencing has increased the likelihood of early HSP diagnosis, especially when there is a positive family history. In the absence of affected family members, however, the diagnosis depends mainly on the combination of clinical history, imaging, genetic testing, and electromyography to rule out other diseases that present similarly [11]. Recognizing clinical and typical imaging features is pivotal for early diagnosis and avoiding unnecessary investigations.
Current treatment for HSP in both children and adults is purely symptomatic [12]. Pharmacological therapies include baclofen, tizanidine, dantrolene, and botulinum injections. Physical therapy is sometimes useful. Surgical procedures to lengthen the heel cord have been used to improve the gait [7].
2 Methods
We present the clinical manifestations and imaging features of two siblings with childhood-onset, autosomal recessive HSP. Genetic testing confirmed compound heterozygous SPG11 mutations, which follow an autosomal recessive inheritance pattern. Our Institutional Review Board for Human Research does not review case reports.
3 Patient Discussion
Patient 1: This 27-year-old man presented with chronic progressive difficulty with walking and poor coordination which began during childhood. At age 21, he stopped driving began using a cane to walk. He also reported urinary incontinence and a poor memory. As a child he experienced developmental delay and cognitive impairment for which he received special education, physical, speech, and occupational therapies. He was born prematurely at 30 weeks gestation via emergency Caesarean section and had a 1-month neonatal intensive care unit stay without intubation.
His examination was notable for spasticity, more in the legs than the arms, with hyperreflexia and a spastic gait. He was seen by two adult neurologists in the past and was diagnosed with CP and spasticity.
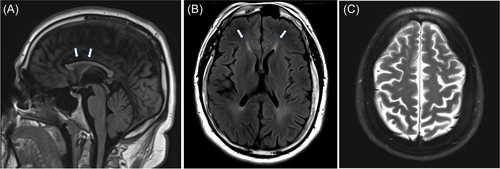
Previous investigations included magnetic resonance imaging (MRI) of the brain, which showed thinning of the corpus callosum (TCC), periventricular signal changes, and mild atrophic brain parenchyma (Figure 1A–C). Electromyography was normal. Neurosychometric testing in 2020 showed borderline intellectual functioning with an IQ score of 68. He was given a trial of baclofen, cannabidiol, and tizanidine for spasticity and transiently improved. In 2022, he was seen by a pediatric neurologist. Based on the history and brain MRI features, including the “ears of the lynx” sign, HSP was suspected. A subsequent Invitae HSP panel showed two heterozygous pathogenic variants, deletion (exons 31 to 34) and c733_734del (p. Met245Valfs*2) in the SPG11 gene (NM_025137.3).
Patient 2: The 23-year-old sister of patient 1 was evaluated after her brother's HSP diagnosis. She first noticed difficulties with walking and balance at age 20. Since then, her symptoms have progressively worsened, with associated leg stiffness and cramps that interfer with her daily activities. She does not currently need mobility aids. She also reports frequent urinary incontinence and poor memory. Her past medical history was notable for cognitive impairment involving multiple domains. She was born at term and met all her early milestones on time.
Her examination was notable for atrophy of the small hand muscles, mild leg weakness, increased tone in the lower more than upper extremities, hyperreflexia, bilateral ankle clonus, bilateral extensor plantar reflexes, and an ataxic wide-based gait.
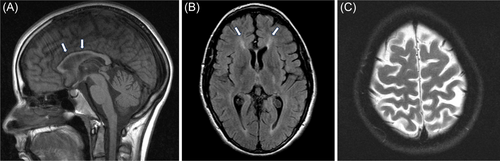
MRI showed mild confluent T2 signal changes of the periventricular white matter of both hemispheres with mild global parenchymal volume loss and TCC (Figure 2A–C). An Invitae HSP panel revealed the same gene variants as her brother with SPG11.
Metabolic studies in both patients showed normal peroxisomal fatty acid profile, copper, lactic acid, thyroid stimulating hormone, free T4, ammonia, homocysteine, methylmalonic acid, and vitamin B12. Eye evaluation was not completed in either patient.
4 Discussion
HSP 11 is the most common cause of autosomal recessive HSP in the United States. It is due to the biallelic loss of function of SPG11, which encodes the protein spatacsin [13]. It is involved in lysosomal function and axonal development. Defects in this protein cause accumulation of glycosphingolipids and gangliosides, as well as dysfunction in organelle trafficking [12], ultimately axonal degeneration, and the development of HSP. In this case series, we report two siblings with typical clinical and imaging features of HSP 11 but with delayed diagnosis and misdiagnosis of CP in one of the siblings.
Both of our patients exhibit clinical features that are similar to those of previously reported individuals with HSP 11. HSP 11 usually presents as a complicated form of disease, with an average age of onset at middle childhood to early adulthood, although it can manifest anytime between infancy and late adulthood. The symptoms typically begin with gait impairment, followed by spasticity and weakness of the legs. As with our patients, developmental delay or learning disability can precede the motor symptoms. Various other central and peripheral nervous system manifestations have been described, including cognitive decline, pseudobulbar involvement, cerebellar signs, and optic atrophy. It can also result in pes cavus and scoliosis [14]. HSP is a progressive disease, with most affected individuals becoming wheelchair-bound 1 or 2 decades after disease onset [15, p. 11].
HSPs can mimic other conditions that feature spastic paraplegia, especially CP, and lead to misdiagnosis [10]. This highlights the importance of considering HSP as a differential diagnosis in patients with CP. The clinical picture of the first patient, including slow progression, positive family history, presence of complex symptoms, and ears of the lynx sign on brain MRI, is inconsistent with acquired injury and guided us to pursue an HSP diagnosis with the genetic study. Recent studies comparing HSP with CP have distinguished the subtle onset yet progressive nature of HSP from the static picture patients with CP usually present. Although patients can have speech delay and learning disabilities concurrently or previously, it is the motor symptoms that usually brings them to clinical attention, as was true with both our patients. Unfortunately, the motor symptoms are subtle at the disease onset, progressive but slow, which can make HSP hard to recognize at the beginning of the disease course. Patients only seek medical attention when the symptoms become bothersome, which significantly delays interventions. It is unclear exactly when the motor symptoms started in both of our patients. It is clearly delayed on seeking medical attention based on the history. Therefore, a history of delayed early developmental milestones and learning disabilities presents an opportunity for early diagnosis of HSP by keeping a high index of suspicion of this disease and looking for other features of HSP, such as a positive family history and a detailed physical examination looking for subtle spasticity, gait imbalance, and typical imaging features. In fact, our second patient did not even seek medical attention until her brother was diagnosed with HSP.
Both our patients presented with typical imaging features of HSP 11. HSP 11 accounts for up to 70% of the HSP patients, with TCC and developmental delay. Other HSP subtypes can have TCC, including SPG4, 7, 15, 18, 21, 35, 46, 47, 49, 50, and 54. They are classified as HSP with TCC (HSP-TCC) [16]. Other characteristic brain MRI features of HSP 11 include brain parenchymal atrophy, T2 signal abnormalities of the periventricular white matter, specifically involving the forceps minor resembling ears of the lynx [14], which is considered highly suggestive of HSP 11 although not pathognomonic as it has been frequently reported in HSP 15. It is interesting to know that HSP 11 and HSP 15 are clinically indistinguishable [17]. This is because spatacin (encoded by the SPG11 gene) forms a complex with proteins ZFYVE26 (encoded by SPG15) and AP5Z1 (encoded by SPG 48) [10]. Apart from HSP 11 and HSP 15, other differentials for the ears of the lynx sign on MRI include HSP 67, AP-4–associated HSP, and Marchiafava–Bignami disease associated with alcohol abuse and malnutrition [18-20]. Based on the findings in our patients and those in earlier reports, the ears of the lynx sign should suggest HSP 11, even in the early stages of the disease. The T2 hyperintensity changes and the brain atrophy progressively worsen, but it is unclear how early the ears of the lynx sign can be detected. This might be an important question to study as the sign might promote earlier diagnosis of HSP 11 and HSP 15.
5 Conclusions
We describe two siblings with identical SPG11 compound heterozygous gene variants who presented with typical clinical features but with different progression rates and different complex symptoms. Both exhibited almost identical imaging findings of HSP 11, including the periventricular white matter changes involving the forceps minor that resemble the ears of the lynx. We highlight the importance of considering HSP in patients with variable clinical presentations, including individuals previously diagnosed with CP, as the management, prognosis, and anticipatory guidance for the two conditions differ. Clinicians should have a high index of suspicion for HSP in patients with gait abnormality, increased lower extremity spasticity, and frequent falls with clumsiness in the context of pertinent negative signs. Clinicians should also consider HSP in patients with developmental delay or learning disability who have a family history of spastic paraplegia or exhibit subtle upper motor neuron signs. Recognizing the typical neuroimaging features of HSP, especially the ears of the lynx sign, can help in early diagnosis of HSP 11.
Author Contributions
Qingqing Wang: writing – original draft, data curation, methodology, resources. Manikum Moodley: validation, writing, review and editing, data curation, supervision, and conceptualization.
Acknowledgments
We thank Dr. Chang Ho of Dell Children's Medical Center for his help with high-quality brain MRI images.
Conflicts of Interest
The authors declare no conflicts of interest.




