Pyridoxine-dependent epilepsy: Current perspectives and questions for future research
Abstract
Pyridoxine-dependent epilepsy (PDE) was historically defined by a dramatic clinical response to a trial of pyridoxine and the re-emergence of seizures after withdrawal of pyridoxine. Research conducted over the last seven decades has revealed that the phenotype of PDE results from multiple genetic disorders, and the most common disorder, PDE-ALDH7A1, is caused by a deficiency of an enzyme involved in lysine metabolism. PDE-ALDH7A1 is characterized by more than epilepsy, as many patients have abnormalities of brain development, and most patients have intellectual and developmental disability. Treatment aimed at the underlying metabolic defect, in addition to pyridoxine supplementation, has improved clinical outcomes. Recently discovered biomarkers and genetic testing allow for the diagnosis of PDE-ALDH7A1 without the need of a pyridoxine trial and hold the promise for newborn screening. Despite these many advances, PDE-ALDH7A1 remains a clinical and biochemical conundrum. The increasing use of model systems and an international collaboration of clinician-scientists are among the reasons to be optimistic that these questions will be answered in the near future and that the clinical outcomes and quality of life will continue to improve for patients with PDE-ALDH7A1.
Pyridoxine-Dependent Epilepsy (PDE): Essential Clinical Features and Historical Aspects
First reported by Hunt and colleagues in 1954,1 PDE is frequently described as the prototypical metabolic epileptic encephalopathy that is unresponsive to antiseizure drugs, but which is generally successfully treated with a vitamin or another cofactor. These patients commonly present with medication-resistant neonatal seizures that may lead to status epilepticus and that come under control only with the addition of pharmacologic doses of pyridoxine (vitamin B6) to their treatment regimen.2-4 Concomitant improvement in the electroencephalogram (EEG) may occur as well, particularly if the patient is experiencing ongoing clinical seizures and EEG monitoring is conducted at the time of pyridoxine administration (Figure 1).6 The long-term treatment of these patients requires daily supplementation of large (i.e., pharmacologic, not physiologic) doses of pyridoxine.2-4 Hence, these individuals are dependent on the vitamin; they are not deficient. Indeed, clinical pyridoxine deficiency is rare, with an incidence of 0.23 cases/100 000 person-year in US military personal;7 significant deficiency is associated with irritability, abdominal pain, insomnia, weakness, and difficulty with ambulation.8 In childhood, the most often cited example was iatrogenic pyridoxine deficiency secondary to an error in the manufacturing of infant formula, which led to an epileptic encephalopathy quite similar to the neonatal presentation of PDE.9, 10 Importantly, a pyridoxine deficiency may be induced by treatment with isoniazid, and pyridoxine is the antidote for isoniazid overdose.11
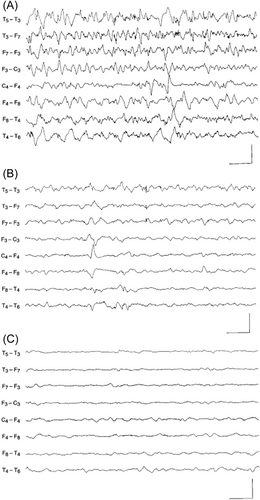
Prior to the discovery of the genetic etiologies of the now three established forms of PDE, the disorder could only be clinically proven by withdrawing the vitamin from a patient who had been successfully treated, in order to demonstrate that clinical seizures would reemerge, generally within a few days, and then be controlled once again by reintroducing the vitamin supplement.2-4 Now, as clinicians have become more familiar with PDE, many patients are treated proactively with pyridoxine, and the diagnosis of the disorder is demonstrated either through the detection of biomarkers and/or by genetic testing.3 In addition, with the availability of targeted genetic screening for epileptic encephalopathies, some patients with PDE are diagnosed prior to institution of pyridoxine treatment. An important distinction to mention is that some patients whose seizures were controlled after the addition of pyridoxine do not have a reappearance of clinical seizures after the withdrawal of the vitamin supplementation. These patients are deemed to be pyridoxine responsive,12 and it has therefore been suggested that the vitamin may have some intrinsic antiseizure effects. For example, the clinical efficacy of pyridoxine in treating seizures in patients with epilepsy due to mutations in KCNQ2 and in some patients with infantile spasms has been reported.13, 14
The Three Established Forms of PDE
Since the initial description of PDE in 1954, additional clinical reports of patients with PDE have appeared, together with a rapidly increasing number of publications describing the natural history, imaging characteristics, neurophysiological features, phenotypic variations, genetic and biochemical features, and treatment recommendations. In a review of the Medline database, between 1954 and 2022, 333 papers concerning PDE have been published, with 72% of the papers appearing since 2005. It is now established that there are at least three forms of PDE (Table 1). The first type of PDE to be molecularly characterized, in 2005, is associated with biallelic pathogenic variants in the PNPO gene, which encodes pyridox(am)ine 5′-phosphate oxidase (OMIM 610090).15 This condition is designated as PDE-PNPO, or PNPO deficiency, and an important feature of this disorder is that approximately 60% of affected patients require treatment with pyridoxal-5′-phosphate (PLP), the biologically active form of pyridoxine, rather than pyridoxine.16 A second form of PDE, due to biallelic pathogenic variants in PLPBP, which encodes PLP homeostasis protein (PLPHP), was discovered in 2016 (OMIM 617290).17 PLP binds to PLPHP, but little is known about the function of this protein.18 Sandwiched between the discovery of these two disorders was the 2006 demonstration of the biochemical and genetic etiology of the most thoroughly described and studied form of PDE. This pyridoxine-dependent metabolic epileptic encephalopathy is due to biallelic pathogenic variants in ALDH7A1, the gene that encodes the enzyme α-aminoadipic acid semialdehyde (α-AASA) dehydrogenase (OMIM 266100).19 This condition is now designated as PDE-ALDH7A1.3 It should be stressed that while PDE-ALDH7A1 is the best studied form of PDE, the specific form of PDE that affected the first PDE patient described by Hunt and colleagues in 1954, along with many additional patients reported through the latter half of the 20th century, is not established. PDE-ALDH7A1 will be the topic of this review, which will focus on clinical, biochemical, and genetic features of the disease, with a particular emphasis on recent developments and ongoing research questions.
| Disorder | Gene | Chromosomal location (hg38) | Function |
|---|---|---|---|
| Pyridoxine-dependent epilepsy | ALDH7A1 | Location: 5q23.2 (chr5: 126 541 841–126 595 219) | Lysine degradation |
| Pyridoxamine-5′-phophate oxidase deficiency | PNPO | Location: 17q21.32 (chr17: 47 941 571–47 949 308) | Catalyzes the synthesis of PLP |
| Pyridoxal phosphate homeostasis protein | PLPBP | Location: 8p11.23 (chr8: 37 762 645–37 779 768) | Homeostatic regulation of PLP |
- Abbreviation: PLP, pyridoxal-5′-phosphate.
Clinical, Genetic, and Biochemical Aspects of PDE-ALDH7A1
Natural history
PDE-ALDH7A1 is inherited in an autosomal recessive manner and is a rare cause of epileptic encephalopathy. Over 200 affected individuals have been reported either in the literature or through rare disease patient registries,2, 4, 20-25 and the estimated birth incidence is 1:64,352.23 The classic neonatal presentation has been well-described in textbooks for several decades. These infants develop medication-resistant seizures within the first several days of life, with some mothers having reported unusual rhythmic fetal movements concerning for intrauterine fetal seizures. In some instances, affected newborns have signs suggestive of birth asphyxia, which can lead to a missed or delayed diagnosis.4, 21, 26-28 A variety of clinical seizure types have been described, including partial, generalized, atonic, myoclonic events, and epileptic spasms. In many instances, patients develop status epilepticus, which may be clinically obscured by the administration of multiple antiseizure medications, but seizures persist electrographically.2, 4, 21 Patients not yet treated with pyridoxine may have waxing and waning encephalopathy, and periods of recurrent seizure activity may be preceded or accompanied by unusual eye movements or facial grimacing.29 A variety of atypical presentations have been described, including onset outside of the neonatal period (up to three years of age and rarely presenting in adolescence or later20, 21, 30-32); seizures that initially respond to antiseizure medications but become intractable; seizures that initially do not respond to the initial administration of pyridoxine but are subsequently controlled with pyridoxine treatment; and patients who have a prolonged seizure-free interval (up to five months) after a trial of pyridoxine discontinuation.2, 4, 21
The natural history of patients with PDE-ALDH7A1 is variable, both with respect to control of seizures and intellectual development. While the sine qua non of PDE is that the affected patient's seizures only come under (often dramatic) control once pyridoxine is added, there are patients who, over time, may require one or more anti-seizure medications.2, 4 This may occur in patients with either the classic neonatal presentation or atypical manifestations. Approximately 75% of patients have some degree of neurodevelopmental disability, ranging from mild to severe intellectual disability, autistic features, and other behavioral problems.2-4 Verbal skills, particularly expressive language, tend to be more impaired. Patients with later-onset of seizures and those with a prompt early diagnosis and treatment tend to have a better prognosis, but this is not always the case.2, 4, 30, 33 This variable natural history may also be due, in part, to associated brain dysgenesis, unknown genotype–phenotype correlations, and levels of one or more toxic metabolites.
Associated developmental features
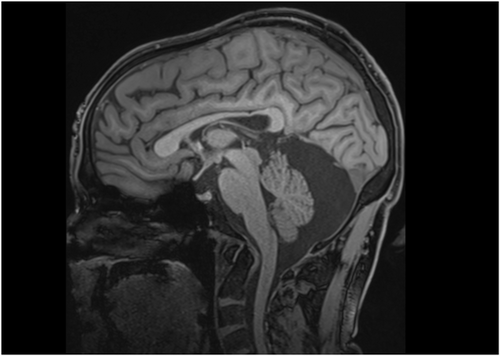
Patients with PDE-ALDH7A1 may have congenital and/or progressive abnormalities of brain development. Thinning of the posterior corpus callosum, primarily the isthmus (Figure 2), is universal and can be quantitatively demonstrated via magnetic resonance imaging (MRI) geometric morphometry.34, 35 In addition, mega cisterna magna and other posterior fossa abnormalities have been noted in approximately 20% of patients (Figure 2).34 Uncommonly, heterotopia and other forms of cortical dysplasia have been demonstrated with imaging and neuropathological studies.36-38 Obstructive hydrocephalus and progressive ventriculomegaly (hydrocephalus ex vacuo, presumably due to late diagnosis and/or poor seizure control) have been described.39 Other developmental abnormalities include congenital cataracts40 as well as variable pattern of facial dysmorphism that includes hypertelorism, depressed nasal bridge, epicanthal folds,41 high hairline, pointed chin, full eyebrows, and broad nasal root (Gospe, unpublished observations).
Biochemical pathophysiology and genetics
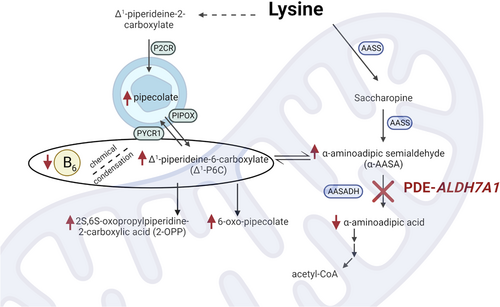
PDE-ALDH7A1 is a metabolic encephalopathy due to an inborn error of lysine catabolism. Biallelic pathogenic variants in ALDH7A1 result in absence or dysfunction of α-AASA dehydrogenase (also known as antiquitin), the enzyme that oxidizes α-AASA to α-aminoadipic acid.19 The underlying pathophysiology of PDE-ALDH7A1 is due to an accumulation of several intermediate metabolites in both the pipecolic acid and saccharopine branches of the lysine catabolic pathway, including pipecolic acid, α-AASA, and Δ1-piperideine-6-carboxylate (Δ1-P6C) (Figure 3). Through a Knoevenagel condensation reaction, Δ1-P6C binds and effectively deactivates PLP, the cofactor that is required for the function of more than 160 enzymatic activities in human cells.42 Dysfunction of one or several PLP-dependent enzymes likely contributes to the epileptic encephalopathy experienced by these patients. In particular, dysfunction of glutamic acid decarboxylase, which converts excitatory glutamic acid to inhibitory gamma-aminobutyric acid (GABA), was first suggested in 1960 by Scriver,43 and reduced GABA synthesis has been demonstrated in cultured fibroblasts from a PDE-ALDH7A1 patient.44 Reduced activity of this enzyme would result in an excitatory/inhibitory imbalance that could underly the epileptic encephalopathy. It is a stark coincidence that while these patients are not pyridoxine deficient, they have a secondary PLP deficiency, thereby requiring large daily supplements of pyridoxine.
Biomarkers
The diagnosis of PDE-ALDH7A1 is now facilitated through the detection of biomarkers in biological fluids and the confirmation of pathogenic variants through testing of the ALDH7A1 gene. Assays for α-AASA45 and pipecolic acid46 can be conducted on urine or blood, and if elevated in a patient with an epileptic encephalopathy would suggest a diagnosis of PDE-ALDH7A1.3 It must be stressed that these metabolites are not specific, as α-AASA can also be elevated in patients with molybdenum cofactor deficiency and isolated sulfite oxidase deficiency,47 and pipecolic acid can be elevated in patients with peroxisomal disorders.48 In addition, in older patients with PDE-ALDH7A1 treated with pyridoxine, pipecolic acid levels may normalize.49, 50 Therefore, specific confirmation of PDE-ALDH7A1 requires an evaluation of the ALDH7A1 gene, generally via both sequence analysis, which will detect >95% of pathogenic variants, and deletion/duplication analysis that will detect <5% of pathogenic variants.23 Evaluation of ALDH7A1 can be done through specific gene testing, multigene panels, and comprehensive genomic testing. The common c.1279G>C (p.Glu427Gln) variant in exon 14 accounts for approximately 33% of the pathogenic variants. Importantly, homozygous c.1279G>C variants have been reported in both patients with classic neonatal-onset PDE-ALDH7A1 and late-onset disease.20-23
The identification of these biomarkers and the availability of genetic testing was critical to the understanding of PDE-ALDH7A1. As we have already discussed, there are multiple genetic causes of PDE that may present with a similar seizure phenotype, and each genetic cause has unique implications for treatment.51 Most notably, establishing PDE-ALDH7A1 as a disorder of lysine catabolism had distinct treatment implications that will be discussed later. Despite the diagnostic implication of elevated pipecolic acid and α-AASA/Δ1-P6C, these biomarkers also had some limitations. Importantly, α-AASA/Δ1-P6C is relatively unstable at room temperature, which requires samples to be frozen prior to shipment to diagnostic laboratories.52-54 This may limit the availability of biochemical testing, and makes the possibility of extending these biomarkers to newborn screening impractical.
In 2019, the metabolite 6-oxo-pipecolate (6-oxo-2-piperidinecarboxylic acid) was identified in children and adolescents with PDE-ALDH7A1.55 Previous studies in Penicillium chrysogenum demonstrated that 6-oxo-pipecolate was a product of the lysine biosynthetic pathway.56, 57 The main benefit of 6-oxo-pipecolate over existing biomarkers was the relative stability at room temperature.55 Shortly thereafter, a second novel biomarker was identified: 2S,6S-/2S,6R-oxopropylpiperidine-2-carboxylic acid (2-OPP).58 Although the role of 2-OPP in lysine degradation is less clear, infrared ion spectroscopy suggests 2-OPP may result from the reaction between Δ1-P6C and acetoacetate.59 Similar to 6-oxo-pipecolate, 2-OPP is relatively stable at room temperature and may have implications for the underlying pathophysiology of disease58 (Figure 3).
Potential for newborn screening
As discussed above, new biomarkers were initially investigated to pursue metabolite-based newborn screening. Although rare, it appears as though newborns with PDE-ALDH7A1 have had fatal status epilepticus before treatment with pyridoxine could be initiated.21, 37, 38 Additionally, treatment with both pyridoxine and lysine reduction therapies in the first six months of life has been associated with significant developmental improvements (see below).24 Therefore, the delay in diagnosis and treatment may be the most significant contributor to mortality and morbidity in this treatable disorder, and suggests that PDE-ALDH7A1 may be an ideal candidate for newborn screening.60 Indeed, it was recently selected as a top priority for newborn screening by members of the US Pediatric Epilepsy Research Consortium.61
The first study focused on newborn screening measured α-AASA and Δ1-P6C in residual dried blood spots, although neither metabolite was detected in unrefrigerated samples.62 Pipecolic acid has also been proposed as a primary biomarker for newborn screening of PDE-ALDH7A1.63 The lack of stability (α-AASA/Δ1-P6C) and concerns about sensitivity and specificity (pipecolic acid) make these biomarkers less than ideal. Both 6-oxo-pipecolate and 2-OPP are promising biomarkers for newborn screening and have been elevated in dried blood spots from affected patients.55, 58 These pilot studies are encouraging, including one study documenting elevated 6-oxo-pipecolate in a 33-year-old residual dried blood spot (Woontner et al. in review), although larger studies are still needed before newborn screening of PDE-ALDH7A1 will be widely adopted.
Treatment of PDE-ALDH7A1
Initial recommendations for the diagnosis, treatment, and follow-up of patients with PDE-ALDH7A1 were published in 2011,64 and treatment recommendations were updated after the first study using a lysine-restricted diet.65 In 2021 the first consensus guidelines for the diagnosis and management of PDE-ALDH7A1 were published by the International PDE Consortium (www.pdeonline.org).3 Recommendations were based on several individual factors such as the age of the patient and the severity of the phenotype. The authors were transparent about the relatively low level of available evidence, and many of the recommendations relied on expert consensus.
Pyridoxine supplementation
PDE is historically defined based on the resolution of seizures following treatment with pharmacologic doses of pyridoxine,1 and treatment with pyridoxine remains central to the treatment of the epilepsy that has defined this disorder. The importance of vitamin B6 supplementation is highlighted by the recurrence of seizures following pyridoxine withdrawal66-70 and case reports that describe patients who died before pyridoxine was administered.21, 37, 38 As noted above, patients with PDE-ALDH7A1 have a secondary deficiency of PLP most likely due to a Knoevenagel reaction between the accumulating Δ1-P6C and PLP.19 Treatment with pyridoxine, as opposed to PLP, is recommended for several reasons. There is an association between PLP supplementation and cirrhosis, including at doses usually required for successful treatment of seizures.71, 72 It is also worth considering that commercial pyridoxine preparations are relatively easy to obtain and stable, whereas PLP degrades quickly when exposed to light.73 The International PDE Consortium consensus guideline for pyridoxine supplementation by age is listed in Table 2. Note that these pharmacologic doses are substantially higher than the recommended dietary allowance (physiologic doses), which ranges from 0.1 mg for infants to 2 mg for lactating females.74 While there is a risk of peripheral neuropathy when the dose of pyridoxine exceeds 500 mg/day,33, 75 the vast majority of patients with PDE-ALDH7A1 do not require such large doses for seizure control. Some patients may require one or more anti-seizure medications, in addition to the maximum recommended dose of pyridoxine, to achieve control of their seizures.
| Age of patient | Pyridoxine dose range | Maximum daily dose (mg) |
|---|---|---|
| Newborns | 100 mg/day | 100 |
| Infants | 30 mg/kg/day | 300 |
| Children and adolescents | 20 mg/kg/day Range 5–30 mg/kg/day |
500 |
| Adults | 200–500 mg/day | 500 |
- Adapted from 2021 PDE Consortium consensus guidelines.3
There have been many reports of presumed intrauterine fetal seizures in newborns subsequently diagnosed with PDE-ALDH7A1. As there is a 25% recurrence risk for a couple having another affected child, maternal pyridoxine supplementation during gestation has been proposed for these at-risk pregnancies, specifically to help control intrauterine seizures and improve developmental outcome.76 Ideally, plans should be made for the fetus to be delivered at an institution that is equipped to care for a newborn at risk of developing an epileptic encephalopathy, and the baby should receive pyridoxine supplementation until the results of confirmatory biochemical studies and/or gene testing have been obtained.
Lysine-reduction therapies
From the early description of the disorder, patients with PDE were described as having intellectual or developmental disability (IDD).1, 77 More recent studies noted that there was no association between the time of seizure onset or severity and IDD, suggesting the poor developmental outcomes may be due to a separate disease mechanism.2, 25, 78 The discovery that PDE-ALDH7A1 was, in fact, an organic aciduria led to the current hypothesis that accumulating α-AASA (or related) metabolites are neurotoxic and contribute to the IDD phenotype.64 Current adjunct (to pyridoxine) therapies attempt to reduce the nutritional intake of lysine or reduce the transport of lysine and are collectively referred to as lysine-reduction therapies (LRTs).
In a cross-sectional study of seven subjects, van Karnebeek et al. treated patients with PDE-ALDH7A1 with pyridoxine and a lysine-free medical formula and demonstrated a decrease in pipecolic acid, α-AASA, and Δ1-P6C.79 Shortly thereafter, a single patient was treated with pyridoxine and arginine, as the dibasic amino acids lysine, arginine, and ornithine are transported by the same cationic transporter in the intestine, the kidney, and the blood-brain barrier.80-82 Following daily arginine supplementation, the patient had a decrease in urine and cerebrospinal fluid (CSF) α-AASA and improved neuropsychiatric testing.83 In a pre–post design, patients who were initially treated with pyridoxine and lysine-restricted diet had arginine supplementation added to their treatment regimen (also referred to as triple therapy). These patients demonstrated a significant decrease in plasma α-AASA and Δ1-P6C.84
Although not the primary outcome measure in these studies, all of the reports noted improved development in those patients treated with a form of LRTs. A recent cohort study evaluated the association between treatment with LRTs and cognitive outcomes. Cohort assignment was based on treatment at the time of developmental testing, and treatment with pyridoxine and LRT was associated with a nonsignificant increase on developmental testing compared to treatment with pyridoxine alone.24 Notably, treatment with pyridoxine and LRTs in the first six months of life was associated with a significant increase on developmental testing scores,24 which emphasizes both the efficacy of LRTs and the importance of early, if not newborn, diagnosis and treatment.
Proposed Future Research Questions
It has been almost 70 years since the initial description of a newborn in whom seizures ceased following a dose of pyridoxine.1 In 2003, three years before the discovery of the genetic basis of PDE-ALDH7A1, Peter Baxter published a review paper on PDE titled “Pyridoxine-dependent seizures: a clinical and biochemical conundrum” in which he discussed both some contradictory biochemical observations and, importantly, some initial biochemical clues that eventually help lead investigators to the discovery of altered function of α-AASA dehydrogenase in affected patients.85 While our understanding of the molecular mechanisms associated with PDE-ALDH7A1 has advanced significantly over the past two decades, several questions regarding underlying biochemistry, pathology, and phenotypic variability of the disorder remain, all of which are ripe for investigation.
Is there a biochemical signature for PDE-ALDH7A1?
Shortly after the initial description of PDE, it was suggested that the glutamic acid-GABA system was involved in the underlying pathophysiology of the disorder.43 It was known that glutamic acid decarboxylase was a vitamin B6-dependent enzyme,86 although prior to the identification of ALDH7A1 it was clear that glutamic acid decarboxylase was not the genetic etiology of PDE.87 Although it remains unclear, elevated CSF glutamate in patients with PDE-ALDH7A1 further suggests the role of the GABA synthetic pathway in this disorder.88 As noted above, PLP is a cofactor for many critical enzymes, and the impact of PDE-ALDH7A1 on these enzymes remains opaque. Of note, patients with PDE-ALDH7A1 have been initially misdiagnosed as having nonketotic hyperglycinemia, as the glycine cleavage enzyme is also PLP dependent.89, 90 These examples further emphasize the impact of the secondary vitamin B6 deficiency in PDE-ALDH7A1 and highlight the importance of evaluating the role of these secondary enzyme deficiencies in the early course of the disease.
What is the biochemical pathophysiology of PDE-ALDH7A1?
The hypothesis that α-AASA is a neurotoxic organic acid was based on relatively circumstantial evidence.64 Of course α-AASA is the primary substrate of the enzyme α-AASA dehydrogenase and, therefore, is the most proximal metabolite to the underlying defect. Although α-AASA is fairly reactive and may quickly be converted to its dehydration product Δ1-P6C,55 the role of Δ1-P6C and PLP deficiency has been better defined, although the chemical condensation product has only been identified in a single study.19 In a study of brain tissue extract from a patient with PDE-ALDH7A1, the metabolites 6-oxo-pipecolate (29 nmol/g), α-AASA (13.02 nmol/g), Δ1-P6C (8.14 nmol/g), and 2-OPP (5 nmol/g) were present,58 although the presence and relative quantification of these metabolites in brain tissue does support direct causality. It is conceivable that the improvement of metabolomics platforms will continue to identify metabolic derangements in PDE,59, 91-93 and future studies need to address whether these metabolites are directly associated with disease pathophysiology or are simply diagnostic biomarkers. It remains possible that other mechanisms are involved in the natural history of this disease, such as mitochondrial impairment88 or oxidative stress.94
It is important to note that lysine is metabolized through two pathways (Figure 3) that were originally thought to have distinct roles, with the saccharopine pathway being predominant in the liver and the pipecolic acid pathway in the brain. The compartmentalized role of each enzyme was supported by high levels of saccharopine dehydrogenase in the liver, relative absence of saccharopine dehydrogenase in the brain, and low levels of l-pipecolic acid oxidase in the liver and predominance in the brain.95-97 Recent studies using wild-type mice and cultured human astrocytes suggest the saccharopine pathway may have a role in brain lysine metabolism and may even be the predominant lysine oxidation pathway in the brain,98, 99 and a number of therapeutic strategies exclusively target the saccharopine pathway. Although these recent studies are compelling, the role of each pathway in critical cells is still unclear.
Is there a genotype–phenotype correlation for PDE-ALDH7A1?
As noted above, PDE-ALDH7A1 is caused by biallelic mutations in ALDH7A1.19 This discovery provided insight into the mechanism of disease and made genetic testing to diagnose the condition widely available.3 The genotypic spectrum has been well defined,22, 23, 100, 101 and these studies have confirmed the presence of a relatively common mutation (p.Glu427Gln) and allowed for the estimation of disease frequency. Thus far, no genotype–phenotype correlation has been identified. It is possible that this is due to the difficulty in generating longitudinal phenotypic data in rare diseases. An alternative hypothesis is that the phenotype is driven by chronic accumulation of α-AASA or related metabolite. If this hypothesis is correct, the rate of α-AASA accumulation and subsequent rate of developmental regression would have a genotype–phenotype correlation. This may be overlooked due to a clinical bias where most patients are diagnosed or evaluated after the accumulation of α-AASA has reached a clinical threshold.
Why do some patients with PDE-ALDH7A1 require treatment with antiseizure medications?
Despite the often dramatic clinical and electrographic response to pyridoxine, many patients require the use of one or several antiseizure medications to control their epilepsy, and in some circumstances complete control is not achieved.2 There are several explanations for why epileptic seizures may not completely respond to pyridoxine and LRTs. For patients who went undiagnosed and untreated for months to years, brain injury from multiple episodes of status epilepticus together with ongoing metabolic neurotoxicity likely result in brain injury that can result in epileptogenic foci. Neuropathological studies of PDE-ALDH7A1 patients have demonstrated areas of cortical necrosis, gliosis, hippocampal sclerosis, and status marmoratus,36, 37 and similar features have been noted on imaging studies.35, 101 In addition, developmental brain dysgenesis, including cortical dysplasia and heterotopia, have also been demonstrated via neuropathological studies36, 37 and magnetic resonance imaging.35, 101 Treatment of epilepsy emanating from these developmental lesions would likely require the use of one or multiple antiseizure medications.
How does α-AASA dehydrogenase dysfunction result in structural anomalies?
It is perplexing that reduced or absent activity of an enzyme that functions within the lysine catabolism pathway also leads to abnormal development of the central nervous system (partial agenesis of the corpus callosum, mega cisterna magma, cortical dysplasia, heterotopia), as well as less common phenotypic features such as congenital cataracts40 and facial dysmorphism.41 Prior to the discovery of the role of antiquitin in lysine catabolism, it was known that the enzyme functioned as an aldehyde dehydrogenase, and that plant antiquitins played a role in osmoregulation.102 Hyperosmotic stress can be cataractogenic, and it has been suggested that this may explain the development of cataracts in some individuals with PDE-ALDH7A1.40 As far as the development of heterotopia and other forms of cortical dysgenesis are concerned, antiquitin has been localized to radial glia and other nonneuronal cells,36 and dysfunction of this protein is likely responsible for these neurodevelopmental lesions. It is conceivable that antiquitin plays biological roles other than just serving as an aldehyde dehydrogenase. Indeed, it has been noted that specific single nucleotide polymorphisms in ALDH7A1 can modify the prognosis of certain oral cancers,103 while a genome-wide association study noted that ALDH7A1 was a susceptibility gene for osteoporosis.104 The concepts of moonlighting (where a site remote from the active site of the enzyme is recruited to serve a secondary function) and catalytic promiscuity (where the active site catalyzes an adventitious secondary reaction)105, 106 should be explored as potential explanations for why these structural anomalies are components of the PDE-ALDH7A1 phenotype.
Are there characteristic structural features of PDE-ALDH7A1?
Thinning of the posterior corpus callosum and abnormal development of the posterior fossa (Figure 2) are well described in PDE-ALDH7A1, and patients have been noted with dysmorphic facial features. Should these various structural changes be considered as characteristic components of the phenotype? Shape analysis of imaging studies in cohorts of PDE-ALDH7A1 patients have quantified the abnormal structure of the corpus callosum, demonstrating that the finding is present across the age spectrum and independent of the time of diagnosis and initiation of treatment.35 These changes may not always be obvious via a routine review of an MRI scan. As far as posterior fossa abnormalities are concerned, mega cisterna magna, when present, is an imaging finding that is quite evident to a clinician. But are there more subtle posterior fossa structural features that may only be detected via geometric morphometry? What about the facial dysmorphic features that have been described? These should not be out-of-hand discounted as part of the clinical phenotype, as various forms of facial dysmorphism are well known in other inborn errors of metabolism such as Zellweger spectrum disorder,107 hyperosmotic stress,108 and congenital disorder of glycosylation type Ia.109 Documentation of the facial features of PDE-ALDH7A1 patients via 3D photography will help determine if certain dysmorphic features are common.
What components of the PDE-ALDH7A1 phenotype will newborn screening followed by early lysine-reduction therapy improve?
As noted above, advocates for newborn screening emphasize the risk of fatal status epilepticus prior to treatment with pyridoxine and the poor developmental outcome for late pyridoxine-treated patients as well as those treated with LRTs after 6 months of age.60 While some patients have had normal development despite delays in treatment with pyridoxine and without the addition of LRTs,24, 110, 111 there is an association between the onset of initial seizures and developmental outcomes,24, 30 although this association must be due to more than seizures alone. Affected patients who receive antenatal or immediate neonatal pyridoxine treatment often have no clinical evidence of seizures and may still have quite poor developmental outcomes.25, 33, 78 It is possible that other modifiers affect the cognitive phenotype of the disorder. Thus far, studies evaluating the impact of pyridoxine and LRT have focused on either surrogate biomarkers or developmental testing such as IQ. It is unlikely that the addition of LRT would modify the anatomical features noted on MRI such as thinning of the corpus callosum, mega cisterna magna, or cortical dysplasia.34-36 Conversely, further studies should also focus on the impact (both positive and negative) of adjunct therapies on behavioral difficulties25 and general quality of life for patients with PDE-ALDH7A1 and their families.112
How will model systems help resolve some of these questions?
Several cellular and animal models have been used to answer these and other questions. Crowther and colleagues used a combination of human fibroblasts and astrocytes derived from human cortex to study the role of pipecolate and saccharopine pathways in lysine degradation.99 Using l-[α15N]-lysine and l-[ε15N]-lysine, the cellular models suggested the saccharopine pathway is the predominant pathway for human lysine degradation to amino adipic acid. A similar isotope tracer method was used to demonstrate saccharopine is the primary pathway for lysine metabolism in mouse liver, kidney, cerebral cortex, and cerebellum.98 Both zebrafish and mouse knockout models have been developed to further understand both disease pathophysiology and response to treatment. In zebrafish, the aldh7a1−/− fish demonstrate spontaneous seizures leading to death, a phenotype which can be rescued with pyridoxine.113, 114 Of note, exposure to lysine exasperates this phenotype. A morpholino knockdown aldh7a1 zebrafish model resulted in both uveal coloboma and skeletal abnormalities, implicating ALDH7A1 in the development of structures other than the central nervous system.115 Conversely, the Aldh7a1−/− mouse does not have an epilepsy phenotype unless exposed to high dietary lysine.116 These models demonstrate that high dietary lysine is associated with both increased metabolic disturbances and worsening of the epilepsy phenotype. On their own, these results do not support the benefit of LRTs, although these cellular and animal models may be critical for the evaluation of current and future therapies.
Conclusion
It has been close to 70 years since the first description of pyridoxine dependency in an infant with antiseizure medication–resistant epilepsy. In the subsequent years, several genetic causes of PDE have been described where first-line treatment with pharmacologic doses of pyridoxine or PLP typically provide dramatic improvement in the epileptic encephalopathy. PDE-ALDH7A1, the most reported and studied of these disorders, is primarily a disorder of lysine metabolism, and current therapeutic strategies are similar to those developed for other organic acidurias. Patients who are diagnosed in early infancy and promptly treated with the combination of pyridoxine and LRT have significantly improved clinical outcomes. Despite these advances, many questions remain about disease pathophysiology, phenotypic heterogeneity, and response to treatment. Current cellular and animal models hold promise to help further unravel many of these questions. Collaborative efforts, such as the International PDE Consortium (www.pdeonline.org), have aided our understanding of the natural history of the disease and the efficacy of various treatment regimens. Taken together, there is reason to be optimistic that the next few years will include more effective treatments leading to improved outcomes and quality of life for patients with PDE.
Author Contributions
Curtis R. Coughlin II: Writing—original draft; writing—review and editing. Sidney M. Gospe Jr.: Conceptualization; project administration; writing—original draft; writing—review and editing.
Acknowledgments
The authors wish to thank Ann Madhavan and Lynly Beard of the University of Washington Health Sciences Library for their assistance with bibliometric research. This publication was supported, in part, by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R21HD104952. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The authors declare no conflict of interest.




