Aqueous Rechargeable Zn–Air Batteries for Sustainable Energy Storage
ABSTRACT
Accelerating global energy demand and associated CO₂ emissions accentuate the urgent need for sustainable energy storage solutions. Aqueous rechargeable Zn–air batteries (RZABs) have emerged as a promising candidate for renewable energy storage, owing to their inherent safety, cost-effectiveness, and reduced environmental impact. However, despite significant progress in laboratory and pilot-scale research, their large-scale deployment remains uncertain. A comprehensive evaluation of their technological maturity and carbon neutrality is essential to bridge this gap. This perspective critically examines the current status of RZABs, recent technological advancements, and their associated CO₂ footprint, with a focus on overcoming performance limitations and enabling large-scale implementation. We conclude by highlighting practical obstacles, commercialization potential, current market status, and future directions for substantial implementation of RZABs in the pursuit of sustainability.
1 Introduction
Demand for sustainable and clean energy storage solutions has amplified due to the continuous increase in global temperatures, triggering serious environmental concerns [1]. While lithium-ion batteries (LIBs) have contributed towards carbon neutrality in reducing fossil-fuel dependence, they face issues in safety, manufacturing, recyclability, and environmental impact [2]. Aqueous rechargeable zinc–air batteries (RZABs) have received significant interest as an alternative, offering a cost-effective and environmentally friendly energy storage solution with intrinsic safety and minimal environmental footprint [3]. By virtue of their high theoretical energy density (1086 Wh kg−1), light-weight and environmental benefits, RZAB holds promise for commercialization, particularly in small devices, grid-scale storage, backup power, electric vehicles, and defense applications [4]. Unlike LIBs, RZABs circumvent issues tied to the mining and processing of lithium, nickel, and cobalt, offering a low-carbon, recyclable pathway for large-scale energy storage [5]. As industries seek sustainable alternatives to LIBs, the global market for RZABs is poised for commercial growth to address the cumulative demand for sustainable energy storage.
Primary ZABs, first introduced in 1878, offered high gravimetric energy densities (500 Wh kg–1cell, coin-cell configuration), nearly double that of LIBs (350 Wh kg–1cell), and found use in compact electronics such as hearing aids and film cameras. However, their applications remained narrow due to low power densities [4, 6]. Thereafter, multiple efforts have been made to develop rechargeable ZABs using half-open systems with air-breathing cathodes but have faced persistent challenges, including low round-trip efficiency (60-70% vs. > 90% for LIBs), poor durability and cycle life, and material degradation [7, 8]. These limitations underscore the gap between laboratory advances and realistic applications, raising critical questions: can RZABs achieve the environmental and performance benchmarks required for next-generation energy storage, and where should future research be directed to enable real-time application?
Regardless of their commercial success, rechargeable ZABs continue to emerge as a promising and sustainable energy storage solution. This perspective aims to critically evaluate RZABs from a sustainability standpoint, focusing on three key dimensions: sustainability, technical challenges and advancements, and the current market landscape with prospects. We present the potential of RZABs for achieving carbon-neutrality by comparing their environmental impact with state-of-the-art LIBs, considering factors like raw material extraction and processing, production emissions, and recyclability. We also explore the technological advancements in RZABs to enhance their efficiency, rechargeability, and lifespan for commercialization. Further, we examine the evolving market landscape, identifying the key players and ongoing large-scale projects, followed by future research directions required for widespread adoption. By discussing these aspects, this perspective provides a holistic evaluation of RZABs as a sustainable energy storage technology with a markedly lower carbon footprint, paving the way for a clean energy future.
2 Sustainable Energy Storage
Achieving carbon neutrality in energy storage requires sustainability across the entire battery life cycle, from raw material extraction to recycling. While LIBs dominate the current market due to mature and established operation, high round-trip efficiencies, and long lifespan, safety issues and recycling standardization must be overcome to achieve carbon-neutrality [9, 10]. RZABs represent an environmentally benign and economical solution, leveraging zinc's abundance, low cost, recyclability, and lower carbon footprint [11]. The air-breathing cathode architecture reduces material use and environmental impact, while aqueous electrolytes enhance intrinsic safety by eliminating fire risks, making them ideal for backup power, grid storage, and military applications, where safety is critical [12]. With high theoretical (1078 Wh kg−1) and practical (200–400 Wh kg−1) energy densities, RZABs are also well-suited for compact, long-lasting power devices like hearing aids [13-15].
The environmental impact of RZABs compared to LIBs hinges on differences across the battery life cycle. RZABs offer notable sustainability advantages by using abundant Zn, whose extraction is less energy- and water-intensive than Li, Co, or Ni [16]. LIB production requires high-temperature cathode processing and involves complex electrode preparation, emitting 120–250 kg CO2-eq per kWh per battery capacity [17, 18]. For RZABs, the life cycle assessment was performed based on the laboratory scale. CO2 emissions were estimated using a cradle-to-gate approach, where RZABs could yield lower emissions (61.2 kg CO2-eq per 1 kWh of stored energy, based on cumulative energy demands during lab-scale production) due to simplified processing and manufacturing [11, 18]. This estimation provides an idea about their sustainability, and these emissions can be further reduced after considering an upscaled industrial process.
RZABs also benefit from safer chemistries (O2 reactant and aqueous electrolyte) and recyclability, avoiding toxic electrolytes and materials as well as energy-intensive recycling procedures in LIBs [19]. The high Zn concentration in RZABs makes its recovery more economical [20]. Standard recycling procedures including alkaline leaching/electrowinning [21], hydrometallurgy [22], and pyrometallurgy [23] are employed for Zn recovery. Utilizing solar energy further boosts commercial recycling to recover Zn [24], and KOH can be easily separated from water via distillation [25]. Although the theoretical energy density of RZAB is higher, practical values are much lower (200–400 Wh kg−1), the efficiency (60%–70%) and performance are compromised due to sluggish cathode reactions and durability issues, restricting applications to low-power domains [26]. The poor rechargeability, faster degradation, and lower efficiency of RZABs cause high energy waste over the battery's lifetime, while short lifespans increase the frequency of replacement and thus overall CO2 emissions. In contrast, LIBs provide much higher round-trip efficiency and lifespan, supporting a broader range of applications from transportation to stationary storage [27, 28]. These trade-offs highlight the tension between environmental promises and technical readiness for RZABs. The overall factors suggesting sustainability of RZABs (Figure 1a) and LIBs are tabulated in Table S1. In summary, RZABs offer promising environmental advantages over commercial LIBs by virtue of their high geographical distribution and lower environmental impact during material processing and manufacturing. However, it is crucial to consider the performance and lifetime for the long-term sustainability of RZABs.
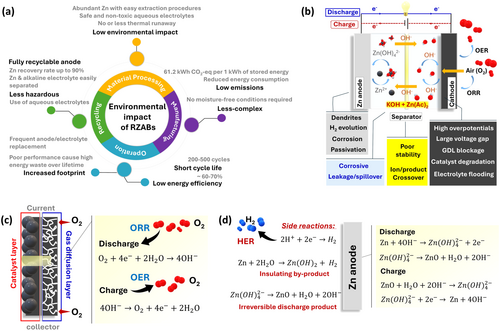
3 Technological Advancements
Despite their advantages, the commercialization of rechargeable zinc–air batteries is hindered by insignificant performance. RZABs comprise a Zn anode and an air cathode immersed in concentrated alkaline electrolyte (6 M KOH), separated by a separator, with each component facing distinct challenges (Figure 1b), that compromise efficiency and durability. At the cathode's triple-phase boundary (Figure 1c), oxygen reduction (ORR) and evolution (OER) reactions occur during discharge and charge, respectively. At anode (Figure 1d), zincate (Zn(OH)42−) ions form upon discharge and convert to insoluble zinc oxide over time, which is reduced back to Zn during charging [3, 29]. Effective electrical recharging demands a bifunctional air cathode and reversible Zn redox chemistry; otherwise, Zn must be replaced to gain mechanical rechargeability [12, 30]. The large charge-discharge voltage gaps stem from high ORR and OER overpotentials, necessitating improved intrinsic cathode activity [31]. Anodes also suffer from parasitic reactions (e.g., hydrogen evolution [HER]) and dendrite formation, causing corrosion and short circuits [32]. These challenges reduce lifespan and pose safety risks, driving research toward technological advancements.
3.1 Improved Cathode Kinetics
Unlike traditional cathodes, air cathodes do not require active material since O2 undergoes reduction and oxidation. However, efficient O2 diffusion and low ORR/OER overpotentials call for well-designed gas diffusion layers (GDLs) and bifunctional catalysts.
3.1.1 Gas-Diffusion Layer
Traditional air cathodes employ hydrophobic polytetrafluoroethylene (PTFE) binders to fabricate carbon-based GDLs [33]. While these GDLs promote O2 diffusion during discharge, they hinder ion transport and retard OER during charging due to strong oxygen adsorption, limiting charge kinetics [34, 35]. Efficient OER requires hydrophilic channels in addition to hydrophobic pathways for ORR. Hydrophilic surface will allow H2O adsorption and facilitate OER, while a hydrophobic/aerophilic microenvironment will facilitate (a) O2 bubble detachment from GDL and (b) O2 diffusion towards the cathode during ORR subsequently [36-38]. Introducing a hydrophilic polyvinyl acetate/carbon black layer between Pt/C + IrO2 catalyst and PTFE-based GDL allowed O2 release during charging via aerophobic interface (contact angle ~153°, Figure 2a), reducing charge voltage by 190 mV and improving energy efficiency by 6% [38]. Similarly, an expanded PTFE (ePTFE) air cathode facilitated gas transport through a “positive bubble diode” mechanism [41], with relatively hydrophobic/aerophilic front (100°) and more hydrophobic/aerophilic back (125°) (Figure 2b) [39]. Bubble diode mechanism is based on the Janus system (unidirectional transport of underwater gas bubbles, i.e., bubble-diode) in fluid dynamics, assisting O2 bubble management and transport during RZAB charging (bubble penetration from less aerophilic side to more aerophilic side) [39, 42]. In contrast, conventional Ni foam and carbon current collectors show reverse bubble diode effect (hydrophobic front facing electrolyte), restricting O2 bubble release [43].
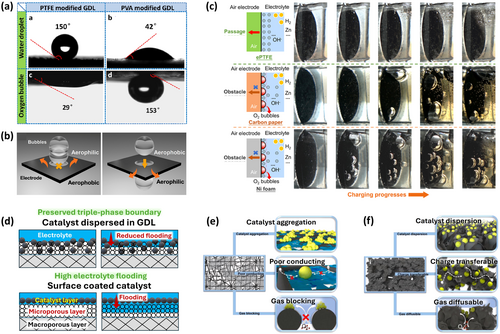
The ePTFE electrode eliminated bubble accumulation due to optimal wettability, enabling rapid O2 diffusion (Figure 2c) [39]. Mitigating electrolyte flooding also enhances cathode kinetics, where in catalyst distribution within GDLs rather than surface coating, preserves the triple-phase boundary and improve discharge kinetics and cycling stability (Figure 2d) [44]. This approach prevents the destruction of porous structure from traditional fabrication procedures (e.g., pressing, sintering) [45]. Solid/semi-solid electrolytes further address flooding but require optimized cathode wettability for practical performance [26, 46]. Other GDL modifications include (a) heat treatment to control hydrophobicity [47], prevent flooding and extend cycle life through modification of functional groups and carbon structure [48, 49]; (b) hierarchical structures, such as porous curly graphene films (PCGF), which improved GDL conductivity and microporosity, yielding 72% round-trip efficiency with Pt/C@PCGF cathode (Figure 2e–f) [40]; (c) use of high surface area substrates (e.g., Ni foam, carbon felt, carbon paper) [50, 51]; and (d) novel fabrication procedures [52, 53]. Overall, cathode kinetics is governed by GDL thickness and porosity, balanced hydrophobic–hydrophilic channels, surface area utilization, and optimized coating/drying techniques to maintain conductivity and structural integrity.
3.1.2 Oxygen Bifunctional Catalysts
Bifunctional catalysts are crucial for enhancing RZAB performance. While benchmark unifunctional catalysts for OER (RuO2/IrO2) or ORR (Pt/C) offer high activity, they are practically limited by cost, scarcity, complex synthesis procedures and compromised stability [54]. Oxygen bifunctional catalysts offer considerable promise for RZABs, yet achieving high activity remains challenging due to different ORR-OER reaction pathways, distinct binding energies of key intermediates (OH*, OOH*), and interdependent OH−/O2 adsorption energies [55]. Current bifunctional catalysts exhibit round-trip efficiencies below 70% due to large ORR-OER overpotentials, obliging continued efforts to enhance bifunctional activity. These large overpotentials arise due to complex multistep reactions, sluggish electron/proton transfer, insufficient catalyst sites, and high energy barriers for intermediate binding/adsorption [56]. Even the benchmark Pt, IrO2/RuO2 catalysts exhibit intrinsic overpotentials over 0.2 and 0.4 V for ORR and OER, respectively [57, 58]. Thus, a bifunctional catalyst with ample proton binding sites, appropriate electronic structure and moderate binding strength for ORR/OER intermediates is essential.
Noble-metal based: Noble-metal based catalysts exhibit high ORR/OER activity by inducing lattice strain, modulating local electronic structures, exposing high-index facets, and regulating adsorption energies of oxygen intermediates [59]. However, they present poor stability due to agglomeration/deformation during prolonged cycling, while their high cost, scarcity, and complex synthesis inevitably increase manufacturing costs, hampering practical use (Figure 3a). Significant approaches to address these issues include partial substitution with transition metals (TMs) [31, 63, 64], incorporation into supports for improved stability [59], and single-atom catalysis [65, 66] for maximum atomic utilization. But achieving high stability and retaining intrinsic activity while minimizing noble-metal content remains challenging. Supported noble-metal catalysts (Figure 3b) on carbon materials (e.g., graphene, carbon nanotubes, carbon fibers, micro-/meso-porous carbons) [67-69] enhance conductivity, prevent aggregation, and tune electronic distribution. Though carbon corrosion under high oxidative potentials necessitates TM-supports (oxides, hydroxides, chalcogenides, phosphides) [70-72], providing not only stability but also OER active sites. Chameleon-like Ru single-atoms grafted on nickel-iron layer double hydroxide (RuSA-NiFe LDH) bifunctional catalyst demonstrated reversible ORR-OER (Figure 3c–d) through dynamic interconversion with RuSA-NiFeOOH, achieving 2400 h of cycling at 10 mA cm−2 [60]. In future, developing multifunctional hybrid supports exhibiting electronic properties of carbon and redox tunability of transition metal is essential. Two-dimensional nanostructures of noble metals also enhance performance by preventing agglomeration and exposing active sites. Pd nanomeshes revealed superior activity (power density ~220.23 mW cm−2) than Pd- and Pt nanoparticles due to hole inner-edge reconstruction, exposure of Pd(111), (200), and (311) facets, nanoporous structure and stability in alkaline media [73]. Alloying noble-metals with TMs further optimizes intermediate adsorption energies via ensemble and ligand effects [65, 74, 75], as seen in Sn–Co/RuO2 trimetallic oxides, where Sn doping regulated the electronic environment of Ru and Co (Figure 3e–f) and optimized *OOH/*OH adsorption/desorption, achieving ultralong cycle life (3312 h at 5 mA cm−2) [61].
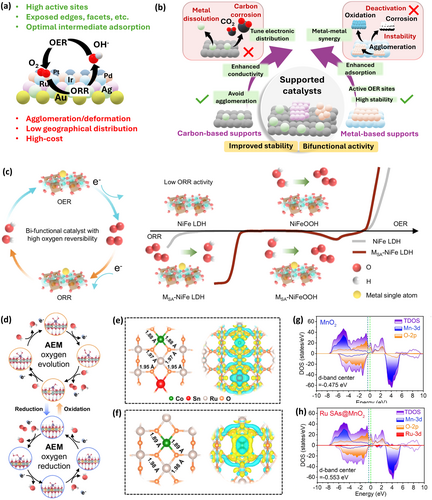
Likewise, a CoCuFeAgRu high entropy alloy (HEA) anchored in N-doped carbon nanosheets boosted RZAB performance (power density ~136.53 mW cm−2), benefiting from Ag-Ru synergistic effects and tailored electronic structure [76]. HEAs are promising due to their disordered local arrangements, unique and tunable electronic structure, which can break the scaling relationships and stabilize intermediate adsorption energies during electrocatalysis [77]. Theoretical screening of alloy composition is required to ensure oxygen bifunctional activity, while the activity can be further improved via conductive supports (e.g., carbon, TM, MXenes), modulating charge distribution and metal-support interactions. Single-atom catalysts effectively reduce the amount of noble metal without compromising the activity due to enhanced atomic utilization. Ru single atoms on MnO2 nanorods (Ru SAs@MnO2) shifted the d-band center negatively (-0.553 eV) compared to MnO2 (−0.475 eV) (Figure 3g–h), increasing charge density at Mn sites to promote ORR while Ru enhanced OER activity [62]. Future directions must emphasize on dual single-atomic sites to enhance bifunctional activity and simplified synthesis procedures in view of sustainability.
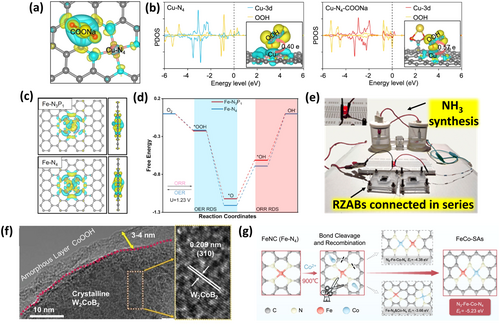
Non-noble metal-based catalysts: Despite the advancements in noble-metal catalysts, sustainability concerns have prompted a shift towards TM-based catalysts (e.g., oxides, sulfides, phosphides, borides) [78-82] for low-cost RZABs with reduced environmental footprint. Their bifunctional activity arises from vacant TM d-orbitals, modulated chemical binding with reactive oxygen intermediates [83]. Strategies such as active site optimization, surface functionalization, defect engineering, and morphology control have been pivotal in enhancing their catalytic performance [84-86]. Integrating multiple metal sites induces synergistic effects, creates bifunctional active sites, and tunes electronic structures, enabling reduced voltage gaps (< 0.8 V), surpassing the benchmark IrO2 + Pt/C catalyst [87, 88]. A Cu–N–C catalyst functionalized with Na-containing groups (CuNa-CF) achieved over 5000 h of battery life (1 mA cm⁻²) due to reduced carbon corrosion, enhanced conductivity through unique electronic structure between Cu-N4 and –COONa (Figure 4a) and accelerated O2 kinetics via strong anchoring of OOH* at Cu-N4-COONa depending on Bader charge transfer (Figure 4b) [89]. Beyond oxides, hydroxides, and chalcogenides, TM phosphides and borides reveal competent bifunctional activity. Electronegative phosphorus shifts the TM d-band center close to the Fermi level [93], increases e− delocalization to induce more positive charges on TM [94], thereby enhancing redox activity. Covalent M-P bonds improve stability [95], while surface oxyhydroxides (M-OOH) and inorganic phosphates reduce OER overpotentials and improve wettability [79, 96]. Fe single-atom catalyst on hierarchical N, P co-doped carbon nanofibers with asymmetric Fe-N3P1-C sites exhibited enhanced ORR/OER performance due to increased Bader charge (7.237e) and thus increased charge density at Fe due to the presence of P atoms (Figure 4c) [90]. Further weak *O and *OH adsorption over Fe-N3P1-C enhanced ORR while lower ΔG (0.89 eV) stabilized *OOH and reduced energy barrier for OER (Figure 4d), respectively [90]. However, monometallic phosphides remain less active than noble metals, necessitating further engineering through doping, defect creation, and heterostructure design [97]. Multi-metallic phosphides demonstrate enhanced performance due to synergistic effects and altered electronic structures [95]. Further, preparing a composite with sulfides, carbon materials, etc., boosts bifunctional activity via enhanced charge-transfer properties [98, 99]. A catalyst derived from organophosphorus complex (1,1-bis(diphenylphosphine)ferrocene (DPPF)) underwent in situ conversion to form Fe-NiCoP nanoparticles confined in heteroatom-doped carbon matrix (NPC), boosting RZAB performance through strong Fe–NiCoP–NPC interactions [100]. Challenges such as surface oxidation and phase separation in multi-metallic phosphides persist [101, 102], highlighting the need for conductive carbon coatings and optimized synthetic routes to prepare stable single-phase materials [103]. TM borides are also promising for bifunctional oxygen catalysis, though their practical use is limited by tedious synthesis involving toxic gaseous precursors and expensive equipment, as well as susceptibility to oxidation [104, 105]. Simplified methods like sonochemical reduction have yielded stable Co2B catalysts with minimal surface oxidation and particle agglomeration [85]. Amorphous CO2B revealed high bifunctional activity via B-2p and Co-3d orbital hybridization and B-O species mediated intermediate adsorption for high-performance RZABs (power density ~500 mW cm−2) powering electrochemical ammonia synthesis for 2 h (NH3 yield: 1.048 mg h−1 mgcat.−1, Figure 4e) [85]. Doping secondary metals further enhances OER and reduces charging overpotentials by decreasing the Bader charges in metal and boron atoms, promoting surface reconstruction and formation of amorphous oxyhydroxide layers (Figure 4f) [91]. Major improvements in the bifunctional activity of TM phosphides/borides require dopant and defect engineering, environment-friendly and controlled synthesis routes to tune morphology and boost mass/electron transport. Single-atom catalysts offer significant opportunities, especially with dual-metal sites. A dual single-atom catalyst (FeCo-SAs) on N-doped graphene nanosheets achieved a cycle life of over 550 h at 10 mA cm−2. The metal synergistic effect transformed Fe-N4 to a more favorable and asymmetric N3–Fe–Co–N4 configuration during pyrolysis (Figure 4g), enhancing adsorption/desorption of ORR/OER intermediates [92]. However, low metal loadings are witnessed for single-atom catalysts, obliging efficient synthesis methods. A plasma-bombing synthesis [106] could achieve high loading of single atoms (2.5 wt%) in a Co single-atom catalyst anchored on N-doped carbon, enabling high ORR and thus discharge performance. Non-noble metal catalysts still require substantial improvements to surpass noble-metal benchmarks. Beyond experimental advancements, theoretical modeling remains crucial for unraveling mechanisms, optimizing catalyst design, and identifying active sites for next-generation bifunctional catalysts.
Carbon-based catalysts: Carbon-based catalysts have emerged as promising bifunctional oxygen catalysts due to high surface area, tunable activity, and scalable synthesis, including green routes utilizing biomass- or waste-derived precursors [107]. However, sluggish oxygen redox kinetics and poor chemical stability at high positive potentials remain challenging.
Heteroatom doping is widely employed to regulate the electronic structure and enhance both catalytic activity and stability [108]. While single-heteroatom doping shows limited bifunctionality, co-doping stabilizes oxygen intermediates via cooperative interactions between p-electrons of heteroatoms. Pyridinic N–B pair doped carbon microspheres (NB-CMS) with hierarchical micropore-mesopore structure achieved ORR and OER bifunctional activity with B@PyN configuration, as evidenced by their position on volcano plots correlating limiting potentials (UL) with ΔG⁎OH and ΔG⁎O − ΔG⁎OH, respectively (Figure 5a–b) [109]. Incorporating e− donating functional groups further mitigates carbon corrosion by reducing the positive charge density [112]. Functional group modification also influences dopant distribution and accelerates oxygen kinetics. In parallel, defect engineering enhances intrinsic catalytic activity by altering the atomic and electronic structure of carbon through vacancy, topological, and grain boundary defects [113]. Hierarchical porous carbons, integrating micropores, mesopores, and macropores, demonstrate enhanced activity and stability in RZABs, where in micropores increase surface area, while meso- and macropores enhance reactant accessibility and mass transport [114]. Interfacial effects further amplify local electric fields and generate active sites. Unlike carbon materials with limited meso- and macropores, N,S co-doped carbon with a hierarchically porous structure (HPNSC) exhibits abundant micro-, meso- and macropores with larger pore volume (1.05 cm3 g−1) and surface area (1261 m2 g−1), with enhanced electrochemically active surface area (ECSA ~ 429 m2 g−1) relative to non-hierarchical NSC (Figure 5c). The larger meso- and macropores in HPNSC expose more active N,S sites to O2, highlighting the role of porous architecture in improving both ECSA and intrinsic activity beyond simple mass transport [110]. But still, achieving benchmark performance will require the development of cost-effective synthesis methods, rational bifunctional site engineering, and strategies to enhance carbon durability by mitigating corrosion and electrolyte carbonation. Heteroatom co-doping strategy, defect and hierarchical pore engineering are the major areas to focus on in the future, along with the development of corrosion-resistant coatings to improve performance and durability in RZABs. Besides, biomass-derived synthesis procedures are critical to improve the sustainability of carbon-catalysts. Metal-carbon composites have enlightened oxygen bifunctional electrocatalysis by integrating active metal sites with tunable carbon structures. While carbon materials exhibit high ORR activity, their limited OER performance needs incorporation of metal sites to induce bifunctionality [55, 115]. Optimizing metal loading on carbon substrates is critical to minimize charge-discharge overpotentials. Heteroatom doping, particularly with nitrogen, improves intermediate adsorption and catalytic activity. Metal-containing N-doped carbon (M-NC) catalysts benefit from strong metal–metal, metal–carbon, and metal–heteroatom interactions, contributing to superior ORR-OER activity [116]. But alternate synthesis methodologies beyond conventional pyrolysis like surfactant-assisted approaches [117], use of low-nucleation rate precursors and controlled electrospinning, must be adopted to minimize agglomeration [118, 119]. Recently, a quasi-phthalocyanine conjugated 2D covalent organic polymer (COPBTC-Co) comprising CoN4+4 sites was synthesized through a pyrolysis-free strategy. Additional N4 atoms could alter the antibonding Co orbitals, weakening oxygen adsorption, thereby enhancing bifunctional activity and battery performance (100 h, 10 mA cm−2) [120]. Further, heteroatom co-doped metal-carbon composites can enhance cathode kinetics in RZABs, for example, trace single-atom Fe anchored on N-, S-codoped carbon (Fe-PNSC) could achieve a high-power density (122.2 mW cm−2) and prolonged cycle life up to 300 h at 5 mA cm−2. This is attributed to the honeycomb-like porous structure (0.71 cm³ g−1), large surface area (957.69 m² g−1), single atom Fe-sites, and heteroatom co-doping (4.66 at.% N and 1.9 at.% S), providing modulated charge distribution and multiple mass transfer channels [121]. Though metal-oxide carbon composites exhibit bifunctional activity, long-term durability can be compromised by metal aggregation. Protective coatings help stabilize these composites during cycling and improve catalytic performance [122, 123]. Metal chalcogenides, phosphides, hydroxides, etc., improve bifunctional activity through enhanced conductivity, strong interactions, and facilitated electron transfer at metal–carbon interface. A graphdiyne-induced CoN/CoS2 heterostructure in N-doped carbon interface (CoN/CoS2/NC) perceived facilitated e− transport, reduced carbon corrosion, and enhanced RZAB performance (voltage gap ~1.1 V at 100 mA cm−2) [111]. This is attributed to π–e−-rich alkyne interactions, pore engineering, and fine-tuned heterojunction (Figure 5d–e) with higher e− density near the Fermi level due to strong CoN–CoS2 interfacial interaction [111]. Similarly, a high entropy alloy (FeCoNiCuMg) carbon nanocomposite with a hierarchical porous structure comprising micro-, meso-, and macropores enhanced the metal active sites utilization [124]. Nonetheless, further research necessitate optimizing metal loading and ensuring their uniform dispersion during catalyst synthesis. Interface engineering through hybrid coordination environments (e.g., metal–heteroatom–carbon interactions, in situ formation of active sites) can drastically boost the cathode activity. Controlled synthesis procedures should also be looked after to avoid metal aggregation, enabling the practical use of metal–carbon composites. However, the complexity of metal-carbon composites results in poor fundamental understanding of bifunctional sites, obliging advanced in situ techniques to elucidate mechanisms. Molecular catalysts show promise, owing to their definite structure, which doesn't require complex techniques to understand the intrinsic activity [125], nonetheless, tuning their activity is challenging. Electrochemical performance of a Co-porphyrin-based catalyst (Co (III) meso-tetra (N-methyl-4-pyridyl) porphyrine) was improved by supramolecular assembly with chemically converted graphene through synergistic (electrostatic/π–π) interactions [126]. But again, molecular catalysts can easily degrade during ORR, due to the presence of oxygen free radicals [127]. Thus, stabilizing molecular catalysts under such environments while screening their structural evolution is crucial.
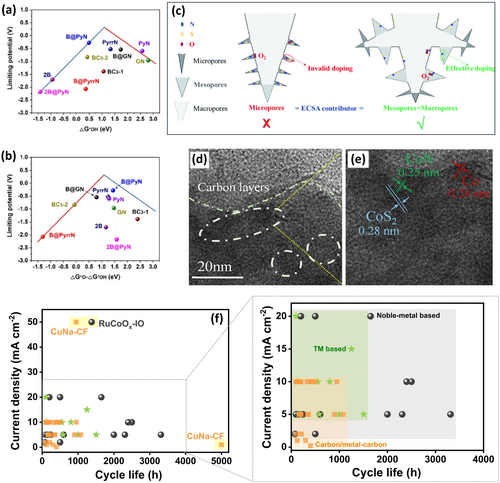
Table S1 and Figure 5f summarize different catalysts with cycling performance exceeding 100 h. A mixture of benchmark catalysts for ORR and OER (Pt/C + IrO2) reveals inferior cycling performance ( < 100 h), where alloying with transition metals holds immense potential. Doping RuO2 with Mn prolonged the cycle life up to 2500 h (10 mA cm−2) [31]. In addition, bimetallic composites (Sn–Co/RuO2: 3312 h @ 5 mA cm−2) [61], single atom catalysts (RuSA-NiFe LDH: 2400 h @ 10 mA cm−2) [60] and HEAs (CoCuFeAgRu: 1650 h @ 20 mA cm−2) [76] show utmost potential to improve the performance of noble-metal catalysts. In contrast, TM-based catalysts show inferior performance than noble-metal counterparts, with TM phosphides being most promising, for example, NiCoP@CoNi-LDH/SSM could operate over 1505 h at 5 mA cm−2 [128]. Tailoring bifunctionality in TM phosphides via doping, alloying, heterostructuring, and morphology tuning as well as employing bimetallic and single-atom configurations, will boost battery efficiency. Metal-carbon composites provide combined benefits from metal centers and carbon. A bimetallic CuNa-CF catalyst ensured a high cycling performance of 5000 h (1 mA cm−2) and 950 h (50 mA cm−2) via C2-Cu-N4 sites (provides stability during OER) and Na (boosts activity of Cu-N4 center) [89]. Further, TM phosphide composite with heteroatom-doped carbon shows massive potential, for instance, a Co/Co2P@NCNT catalyst based Zn-air battery enabled 1080 h of operation at 5 mA cm−2. This summary of the most promising catalysts depicts that noble-metal-based catalysts hold the highest potential to achieve practical performance requiring reduced noble-metal content while retaining the maximum utilization. While numerous efficient, robust, and cost-effective oxygen bifunctional catalysts have been reported, they often fall short of meeting practical performance and sustainability demands. Follow-up efforts combining electrode design and engineering using relatively green synthesis procedures, electrolyte optimization, and mechanistic understanding are essential to realize practical RZABs. Moreover, air cathodes must be evaluated under practical conditions, i.e., high current densities and operating temperatures, to fill the gap between laboratory research and commercial applications.
3.2 Durability Enhancements
In addition to improving cathode kinetics for better rechargeability, the durability of battery should be enhanced by addressing drastic issues at cathode, anode, separators, and electrolyte.
3.2.1 Air Cathode
Air cathode degradation arises from carbonate precipitation, electrolyte flooding, cathode erosion, and GDL blockage (Figure 6a), ultimately shortening lifespan and even battery failure, as confirmed by manufacturing companies (Yardney Electric Corporation [131], Electric Fuel Limited [132]) and mathematical modeling [133]. The main culprit is CO2 interference from the air, inducing carbonate precipitation and blocking O2 diffusion [134]. CO2 removal strategies like adsorption, absorption, membrane separation, and gas cleansing have been explored, though bulky equipment hinders practical use [135]. An electrically rechargeable flow-type Zn-air battery was scaled up with a CO2 adsorption unit, revealing enhanced durability (760 h), though battery failure was attributed to electrolyte flooding rather than carbonate blockage [136]. Integrating CO₂-adsorbing features directly into cathodes offers a more scalable solution. Additionally, carbon corrosion at high oxidation potentials and O₂ bubble accumulation during charging must be addressed. Improvements in electrode design such as (a) polymer coatings to reduce corrosion, (b) engineered GDLs for better electron/mass transport and manage electrolyte flooding, and (c) defect engineering to prevent catalyst degradation, have shown promise to overcome durability issues.
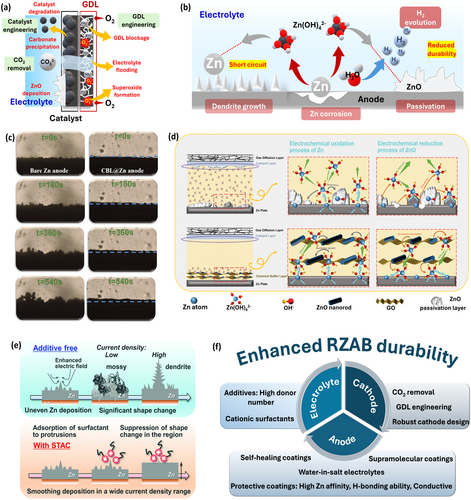
3.2.2 Anode and Electrolyte
At anode, Zn dendrite formation (causing short circuit), parasitic reactions with electrolyte, passivation, and corrosion deteriorate performance and durability, leading to battery failure (Figure 6b). Suppressing dendrites and minimizing side reactions are crucial to extending battery life [137]. Uniform Zn deposition through surface modification and electrolyte engineering helps control dendritic growth by modifying the surface electric field and boosting zincate ion transport. Protective organic/inorganic coatings with high Zn affinity reduce desolvation energy and nucleation barriers, promote uniform Zn deposition and preventing direct contact between Zn and electrolyte, thereby enhancing battery lifespan and safety. This is attributed to the strong
coordination of organic groups with Zn (through H-bonding) to prevent direct contact between the anode and active H2O via replacement of H2O in Zn solvation sheath [138]. While inorganic coatings like TiO2, graphene, etc., mitigate anodic issues via layered adsorption effect and bonding with H2O molecules, their high cost and complexity advance low-cost organic coatings [139-141]. Metal-organic frameworks (MOFs) having high electronegative functional groups can easily form H-bonds with H2O in [Zn(H2O)6]2+ [142], and polymer coatings (e.g., polyvinyl alcohol [143], polyacrylonitrile [144]) also show efficacy but require optimized conductivity, thickness, and wettability. Conductive carbon coatings such as graphene oxide aid e− transfer and reduce Zn dissolution, minimizing anode passivation [140]. A chemical buffer layer (ZnO nanorods dispersed in graphene oxide [GO]) coated Zn anode (CBL@Zn) improved Zn reversibility and enabled long-term cycling of 450 h, attributed to ZnO nanorods limiting nuclei overgrowth and GO regulating precipitation-dissolution behavior of insulating ZnO through adsorption and confinement of zincate ions (Figure 6c–d) [129]. Anion-exchange ionomers can recover Zn during electrodeposition from oxidized Zn discharge products, enhancing durability [145]. Inorganic corrosion inhibitors (e.g., Bi, Pb, Ni, TiO2, Bi2O3, Al2O3, Si(OH)4) suppress HER by increasing its overpotential, but concerns over toxicity, cost, and environmental drive interest in eco-friendly organic inhibitors like polyethylene glycol and polyacrylonitrile, which bind Zn via hydrophilic groups to inhibit side reactions [146, 147]. In the future, supramolecular coatings with biological Zn-binding sites [148], water-in-salt electrolytes [149], and self-healing polymer coatings [150] should be largely focused to promote desolvation and improve Zn reversibility. Electrolyte additives also influence dendritic growth and unwanted reactions. Organic additives (e.g., cetyltrimethylammonium bromide, sodium dodecyl sulfate, polyethylene glycol, thiourea) influence anode surface morphology to reduce dendrites, HER, and Zn corrosion [130, 151-153]. Inorganic additives (e.g., Ni, Pb, CuCl2, CuO, Zn(ClO4)2) promote homogeneous Zn deposition by metal ions with higher reduction potentials than Zn, which can undergo reduction before Zn and tune the electron density at anode [154]. Anti-corrosion surfactants/additives like sodium dodecyl sulfate, sodium dodecylbenzene sulfonate, etc., inhibit ZnO formation and anode passivation [155, 156]. A cationic surfactant (trimethyloctadecylammonium chloride (STAC)) efficiently suppressed dendrite growth via adsorption of ammonium cations onto uneven Zn surface via electrostatic interactions, promoting lateral growth to form a smooth Zn layer ( ~17 μm, Figure 6e) [130]. In summary, the engineering of anode, cathode, and electrolyte altogether, can improve the durability of RZABs for practical applications (Figure 6f).
3.2.3 Separator
Separator stability is critical in RZABs, as dendritic growth can puncture the separator, causing short circuits and failure. Degradation is also linked to the crossover of anode discharge products and deposition at the cathode [157]. Commercial separators, like Celgard, offer mechanical strength but feature large pores that allow zincate crossover [158]. To optimize separators, improvements in ion selectivity, chemical stability, and conductivity are required. Commercial separators often exhibit hydrophobic properties, limiting ionic conductivity. Functionalization of separators, such as sulfonation of microporous Celgard 2320 separator, improved ionic conductivity by 132% (3.52 × 10−2 S cm−1) [159]. An electrospun nanofiber mat-reinforced composite (ERC) membrane prepared by polyvinyl alcohol-impregnated electrospun polyetherimide nanofiber mats prevents zincate crossover without compromising OH− conduction (Figure 7a), as visualized using thymol blue indicator (Figure 7b) [160]. Selective anion exchange membranes (AEMs) improve ionic selectivity by permitting OH⁻ transport while retarding Zn2+ crossover, mitigating ZnO precipitation at cathode. AEMs grafted with N,N-diallylpiperidinium chloride (DAPCl) monomers on modified PPO improved conductivity, alkaline stability, and reduced zincate crossover. A trade-off between power density and zincate crossover is realized, signifying that despite similar power densities of PPO-6CH2-3x and PPO-6CH2-2x AEMs, PPO-6CH2-2x are more durable due to reduced crossover [162].
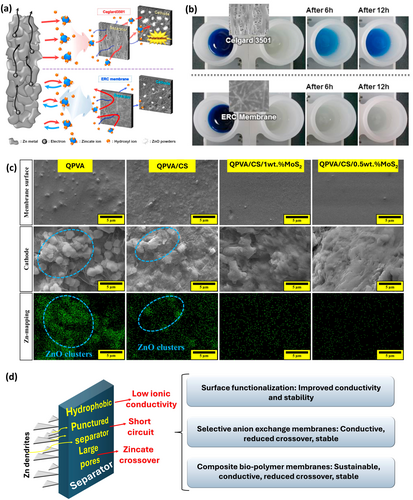
Composite membranes made from affordable biopolymers offer environmental friendliness, stability, and good electrolyte absorption. A quaternized polyvinyl alcohol (QPVA)-chitosan (CS) separator embedded with molybdenum disulfide (MoS2) revealed (a) high conductivity (87.3 mS cm−1) via Grotthus and vehicular mechanisms and surface hopping, enabling OH− transfer and (b) prolonged lifespan over 270 h due to enhanced stability via electrostatic interactions between polycationic polymer and negatively charged MoS2 and reduced zincate crossover, resulting in less ZnO deposition at cathode (Figure 7c) [161]. Despite progress (Figure 7d), separator development for RZABs lags behind, presenting significant room for advancement.
3.2.4 Cell-Configuration
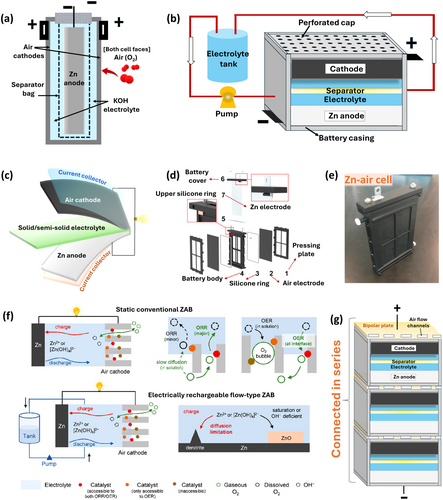
RZABs face durability issues due to anode, cathode, separator, and electrolyte degradation, with the accumulation of solid ZnO discharge products being a key challenge. These discharge products must be removed or reduced back to Zn metal during prolonged cycling. Electric Fuel Limited introduced separator bags (Figure 8a) to recover metal oxides during anode replacement but failed to prevent Zn passivation or reduce electrolyte resistance [165]. A flow-type system (Figure 8b) with continuous electrolyte circulation could address these issues by removing H2 bubbles and ZnO, but the added complexity and manufacturing costs of electrolyte tanks, circulation pumps hamper practical deployment [166]. Additionally, flowing electrolytes also reduce O2 transport and battery durability. Alternative strategies include solid or semi-solid electrolytes (Figure 8c) like alkaline anion exchange membranes, gel polymers, hydrogels, etc. [167-169]. AEMs impede Zn2+ crossover and self-corrosion; however, their limited hydrophilicity causes membrane drying, and quaternary ammonium groups degrade in alkaline environments [170]. Solid/semi-solid RZABs alleviate the leakage risks related to liquid electrolytes due to their enhanced electrochemical stability and mechanical strength, as well as suppress dendritic growth via uniform Zn deposition due to stable ion transfer. Gel polymer electrolytes offer high hydrophilicity and improved ORR/OER kinetics but suffer degradation due to excessive water uptake, rapid consumption of OH− ions, reduced ionic conductivity, and quick evaporation of surface water/liquid phase (alkali climbing [125]). Further improvements in ionic conductivity, water retention, and management remain crucial for real-time application. Cross-linking polymers like polyethylene oxide, polyvinyl alcohol, etc. [171] enhance mechanical stability, while biopolymers (e.g., cellulose [172], chitosan [161]) provide additional benefits of biodegradability, non-toxicity, ease of synthesis, and cost-effectiveness.
In summary, RZABs face significant challenges due to durability issues across all battery components. This perspective emphasizes how to address these issues through targeted component engineering and optimizing cell configuration. Cathode improvements involve the integration of CO2 adsorption sites, advanced electrode designs, and modified GDLs, though scalability remains a concern due to the complexity. Anode can be stabilized through surface modifications, protective coatings, and eco-friendly electrolyte additives. Electrolyte additives can aid in uniform Zn deposition, but issues like flooding and degradation persist. Separators are critical in preventing dendrite penetration and zincate crossover, with functional membranes and biopolymer composites offering improvements, yet the solutions for commercial separators lag behind. Most importantly, the solid ZnO buildup and H2 gas accumulation further hinder the battery's reversibility and safety. Flow-type systems and semi-solid/solid electrolytes show promise but introduce new challenges like complexity, high cost, and membrane instability under alkaline conditions. Despite these hurdles, continued material and design innovations are bringing RZABs closer to commercial viability.
3.3 Scalable Manufacturing
Rechargeable Zn-air batteries offer high energy density and potential to co-exist with LIBs in the market. In terms of scalability, RZABs present a cost-effective alternative due to the abundance and low cost of Zn. RZABs don't require complex dry room conditions, further reducing production costs. Their inherent safety and established recycling technologies cut down CO2 emissions and energy consumption. Aqueous electrolytes eliminate fire hazards, require minimal thermal management, making them suitable for operation in extreme temperatures, unlike LIBs with safety and performance challenges. RZABs can be scaled with mechanical or electrical recharging, with mechanical systems being more mature [173]. Electric Fuel Limited demonstrated an RZAB-powered bus in the late 1990s [174]. In mechanically rechargeable systems, the discharged anode is replaced with fresh Zn, and the electrolyte is refilled after recovering metal discharge products. Battery design is key for scalability, requiring safe and fast anode and electrolyte replacement [173]. One design uses anodes placed in separator bags (Figure 8a), but these bags can puncture with frequent replacements, risking battery failure [165]. A flow-type design (Figure 8d–e) is scalable, but it involves unscrewing bolts for anode replacement [163], making it more suitable for laboratory investigations. Mechanical systems suffer from short lifespans, primarily determined by the performance of the air cathode and separator. To improve scalability, efficient anode/electrolyte replacement, ZnO recovery, and engineering cathodes/separators are critical. In contrast, electrically rechargeable Zn-air batteries face performance and durability issues due to poor Zn reversibility [12]. Improving ORR-OER kinetics with bifunctional catalysts can address these issues. These batteries can be designed as conventional planar cells, flow-cells, and even flexible-type. Conventional planar cells are scalable (same cell as Figure 8b but without electrolyte flow) due to their simplified design. Flow-cells (Figure 8f) can address reversibility issues with continuous electrolyte flow, preventing dendrite formation and Zn passivation, and facilitating solid by-product removal via external filters, but increased complexity and energy use need to be minimized [164]. A Zn-air flow battery built at Técnicas Reunidas delivered 1 kW peak power, but faced issues like electrolyte leakage, increased resistance, GDL flooding, limiting scalability. Although technical feasibility was demonstrated, but the cathodic performance challenges need to be overcome prior commercialization [136]. Flow configurations are used by companies like EOS Energy storage [175], Fluidic Energy [176], and ZincNyx Energy Solutions, but are limited to stationary storage due to space and weight. Flexible RZABs, using solid-state electrolytes to avoid leakage, are also being developed for wearable applications. Large-scale production of flexible RZABs is economical, but their practical deployment is hampered mainly due to performance-related challenges, especially at wide temperatures, reason being the possible degradation caused by electrolyte drying. Binders impose another challenge, requiring self-standing flexible electrodes [177]. On the other hand, bipolar arrangements allow RZABs stacking, increasing output voltage by connecting cells in series/parallel with bipolar plates, comprising air-flow channels instead of external wiring (Figure 8g). This design reduces electrical resistance, amount of anodes, GDLs, and other packaging requirements, enabling high energy densities due to decreased battery weight and cell volume. Nevertheless, maintaining pressure and ensuring high cathode conductivity is crucial in these systems [178]. Both flexible and bipolar designs are scalable but require further research. Additionally, mathematical modeling and artificial intelligence (AI) are essential for optimizing RZAB design and future applications [179].
3.4 Integration With Renewable Energy
Global shift to carbon neutrality has switched the focus on renewable energy sources (e.g., wind, solar) to reduce fossil fuel dependence and CO₂ emissions. However, the intermittency of these renewables reduces their utilization efficiency, necessitating energy storage solutions to store excess energy during peak production and release it when needed [180]. It is imperative to integrate advanced battery technologies with renewable energy to address this loophole. Commercially available batteries like lead-acid and redox-flow have high carbon footprints, low specific energy, and limited lifespans, while LIBs show promise for grid storage but are hindered by safety, cost, and environmental concerns. These shortcomings have paved the way for RZABs, offering higher specific energy, rich resources, and a lower CO2 footprint. Integrating batteries with renewable energy for direct energy conversion and storage is fascinating [181]. For example, solar energy catalyzes OER and can be used in RZABs with photocatalysts to convert solar energy into electrical energy, reducing charging overpotentials [182]. However, poor photocatalytic activity, sluggish OER kinetics, and low solar energy conversion efficiency requires development of multifunctional photocatalysts to improve efficiency above 60% and reduce charge voltage below 2.0 V [183, 184]. Research is focused on optimizing bifunctional photoelectrodes as air cathodes, reducing charging overpotentials, and enhancing durability. A solar-powered Zn–air battery (Figure 9a) utilizing nitrogen-substituted graphdiyne (N-GDY) demonstrated high energy efficiency (90.4%) and reduced charge voltage (1.36 V) under illumination, compared to charging in the dark (1.95 V). This saved 30.3% of energy, however, the complex configuration and poor stability remain critical issues [181]. All-solid-state Zn–air batteries with S-atom-bridged FeNi particles and N-doped hollow carbon nanospheres photocatalyst achieved high power density (126 mW cm−2), reduced charge voltage, and extended lifespan [185]. To address durability issues, electrolyte additives, anode protective coatings, and CO2 inhibitors are essential. Additionally, AI-driven theoretical modeling is crucial for overcoming technical barriers during scale-up and optimizing testing parameters for maximizing renewable energy utilization.
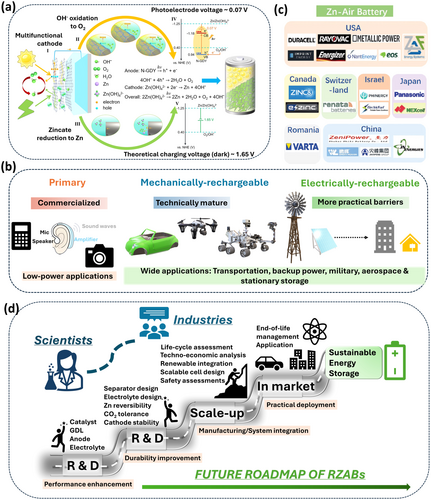
4 Current Market Landscape
Zinc–air batteries have been commercially available since 1930s, initially for railway signals and later for hearing aids in 1970s [186]. Ever since, research is shifted towards broader applications (e.g., grid-scale storage, backup power, electric vehicles, and military/aerospace purposes) by virtue of high energy density, affordability, and environmental sustainability. Zn–air battery market (Figure 9b–c) crossed $1B mark in 2023 and is anticipated to reach $3B by 2036. Primary Zn-air batteries, exhibiting high energy density and low power output, are suitable for low-power applications like hearing aids, small medical devices, calculators, cameras, etc. Patented by G. W. Heise in 1933, these batteries are commercialized by Duracell (Duracell 675 cell: 442 Wh kg−1), VARTA Microbattery, Rayovac, Energizer, Panasonic, NEXcell, and Renata manufacturing companies [4, 186]. They are sealed to avoid contact with air prior to use and must be removed from the device to avoid electrolyte spillage upon failure.
RZABs, particularly those with mechanical recharging, are technically mature (pilot-scale) and feasible [165, 187]. A benchmark work [173] concludes that if Zn-air batteries can achieve 200 Wh kg−1 specific energy and 30–50 W kg−1 specific power, and can be utilized for long-range and low-speed 2/3-wheelers and city buses, while best suited as range extenders for high-power e-vehicles. Manufacturers like Abound developed cost-effective RZABs with Zn regenerative fuel-cell designs, and ZincNyx Energy Solutions is also actively working on RZABs using the same design. However, commercial barriers remain due to issues like increased battery weight, frequent anode replacements, and electrolyte circulation in flow systems.
Electrically rechargeable Zn–air batteries also hold huge promise in transportation and stationary storage applications. NantEnergy announced the deployment of the first scalable RZAB technology in nine countries as an alternative to LIBs in 2018. E-zinc (Canada) also developed a system with separate charge, discharge, and Zn storage operations, claiming up to 80% cost reduction than LIBs for backup power in remote locations [5]. Leading companies like CICenergiGUNE and CEGASA are committed to develop and implement RZABs for both portable and stationary applications. It is only a matter of time before these batteries reach the general market, especially for grid-scale energy storage, where safety and minimal costs are essential. The non-flammability and safety of RZABs make them ideal for backup power, and their lightweight and high energy density suit military and aerospace applications requiring radios, surveillance devices, etc. Despite the potential, RZABs still lag LIBs in cell performance and durability, highlighting the need for ongoing research and development to bridge the gap between current research and commercial deployment.
5 Summary and Prospects
Aqueous rechargeable zinc–air batteries stand at a critical intersection of electrochemical innovation and sustainability imperatives. With their appealing combination of safety, cost-efficiency, and theoretical energy density, RZABs hold promise for low-carbon energy storage, but several persistent performance challenges continue to hinder their practical adoption.
5.1 Technical Challenges
The reversibility of Zn anode remains a core issue, primarily due to dendritic growth and parasitic reactions, compromising efficiency and lifespan. Air cathodes undergo complex degradation, including carbon corrosion, catalyst sintering, and electrolyte flooding. Bifunctional catalysts exhibit sluggish oxygen redox kinetics, while hydrophobic gas diffusion layers retard oxygen release during charging. Aqueous alkaline electrolytes, though facilitating ionic transport, are susceptible to CO₂-induced carbonate precipitation and water loss due to evaporation. Zincate ion crossover through separators contaminates air electrode and degrades catalysts, insufficient resistance to dendrite penetration raises short-circuit risks, and poor chemical stability in alkaline electrolytes causes structural degradation over time. Current research remains largely at the laboratory level, with few demonstrations of pilot prototypes using different cell configurations. Engineering challenges including gas management, sealing, and electrolyte circulation require attention for real-world applications.
5.2 Future Directions
Unlocking the potential of RZABs for sustainable energy systems demands coordinated advances in performance and durability enhancements, manufacturing, system integration, and sustainability assessment for commercialization (Figure 9d). Future research and development efforts should focus on robust, earth-abundant bifunctional oxygen catalysts with hierarchical architectures resistant to corrosion, flooding, and degradation. A deeper understanding of catalyst failure under dynamic cycling, along with tailored electrolyte formulations incorporating additives, corrosion inhibitors, or transitioning to solid/semi-solid forms, is critical. Structured Zn hosts could improve active material utilization and reduce degradation. Strategies for CO₂ tolerance via catalyst/electrolyte design or integrated scrubbing will support open-air operation and enhance durability. Functional separators that suppress zinc dendrite penetration, reduce zincate crossover, and mitigate electrolyte carbonation, all while maintaining high ionic conductivity and mechanical integrity, are the need of the hour. Looking ahead, the life-cycle studies and techno-economic analysis of individual battery components will be essential for large-scale deployment. Transitioning from laboratory cells to modular, scalable systems for grid or off-grid applications requires attention to system packaging, renewables integration, safety assessments, and techno-economic feasibility, accounting for raw material sourcing, manufacturing demands, recyclability, and end-of-life treatment. Taken together, these future directions emphasize the need for holistic, interdisciplinary strategies that move beyond incremental material advances toward system-level optimization and sustainable integration. Aligning scientific innovation with practical constraints and environmental imperatives, aqueous RZABs could position as a sustainable pillar of next-generation energy storage. The future looks promising with growing research efforts dedicated to its development, and now is a critical time for collaboration between research scientists and industry stakeholders.
Conclusions
This perspective surveyed the advancements in RZABs, identified the environmental/operational trade-offs, and assessed the commercial landscape. Although significant hurdles remain, including limited performance and durability, ongoing progress in electrolyte chemistry, electrode architecture, and system engineering is rapidly pushing the field forward. Ultimately, the path to large-scale implementation will depend not only on overcoming technical barriers but also on aligning RZAB development with environmental impact assessments, manufacturing readiness, and economic feasibility.
Author Contributions
Divyani Gupta: conceptualization, writing – original draft, and editing. Zaiping Guo: conceptualization, funding acquisition, and review.
Acknowledgments
We acknowledge the financial support from the Australian Research Council (FL210100050 and IH200100035).
Conflicts of Interest
The authors declare no conflicts of interest.




