Manganese-based polyanionic cathodes for sodium-ion batteries
Yubin Niu and Yanan Zhao contributed equally to this study.
Abstract
Owing to abundant resources and low cost, sodium-ion batteries (SIBs) are sweeping the world at a rapid pace. The cathode is the key to determining the energy density of the battery, and polyanionic compounds have become a representative class of cathode materials due to their stable three-dimensional framework structure, high operating voltage, and good safety. Vanadium-based and iron-based polyanionic compounds are highly regarded in academic communities, but environmental hazards or low energy densities have once overshadowed them in the practical arena. In contrast, manganese (Mn)-based polyanion is one of the potential candidates in terms of cost, voltage, and environmental friendliness, besides, many noteworthy advances have been recorded in recent years. This review summarizes the current research progress of Mn-based polyanions and discusses the challenges they face while looking forward to the future development of such materials and ideas to solve the key problems. It is expected to have a positive effect, that is, to attract more practitioners to focus on the practical way out of such materials.
1 INTRODUCTION
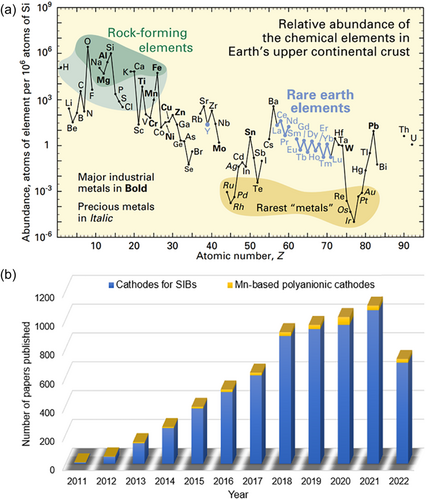
Carbon emissions peak and carbon neutrality goals have endowed new energy and electrochemical energy storage technologies with new vitality and growth points. As a typical representative, sodium-ion batteries (SIBs) have been favored by researchers and institutions in various countries since 2010 due to potential advantages such as abundant resources (Figure 1a) and low cost, and have been assigned high expectations in the field of large-scale energy storage and low-speed or low-end electronic products[2-5]. Many domestic and foreign companies have incorporated SIBs into their capital landscapes, and it is believed that their metamorphosis from sample to product to commodity is in sight[6]. Undeniably, there are still considerable rising margins for SIBs in terms of electrochemical performance, where electrode materials, especially cathode materials, play a crucial role in both controlling costs and determining electrochemical dynamics.
Nowadays, the cathode materials have evolved into five characteristic branches, including layered oxides[7], organics[8], polyanionic compounds[9], Prussian blue, and its analogues[10]. Among them, organics are still in the basic research stage, while layered oxides and Prussian blue and its analogues have been promoted to the product development (upgrading) stage by related enterprises. As far as the most studied layered oxides are concerned, high theoretical capacity is one of their competitive advantages, especially for those containing anion participation[11]. However, the problems of poor structural stability, low ionic conductivity, severe polarization, and storage difficulties during cycling have greatly hindered their rapid layout in practical applications on a large scale[7]. Benefiting from their better stable framework structures, longer cycle lifetimes, faster ionic conductivities, and higher safety, polyanionic compounds, the largest family of cathodes other than layered oxides, have captured the interest of many researchers[12].
Starting with energy density, cycling stability, and rate capabilities, an immersive comparison was carried out by Tarascon and colleagues[13] between the commonly reported layered oxides (P2 and O3 types) and a typical polyanionic compound (Na3V2(PO4)2F3), showing that the latter exhibits unique advantages in all the listed evaluation metrics. Until the major challenges associated with layered oxides such as low redox potential, limited reversible capacity, irreversible phase transition, and moisture sensitivity are addressed, the authors believe that this advantage will remain, which is certainly a strong shot in the arm for practitioners who have been working on polyanionic compound cathodes. Nevertheless, the cost, toxicity, and environmental issues originating from vanadium (V) and fluorine (F) elements are enough to make Na3V2(PO4)2F3 uncompetitive. Therefore, the selection and development of other polyanionic compound cathodes that combine performance, greenness, and cost advantages has become a key challenge to practicality. Given the advantages of environmental friendliness, low price, and high annual production (Figure 1a), the enthusiasm for research on manganese (Mn)-based polyanionic cathodes has been increasing in recent years and many advances have been achieved (Figure 1b). Although Mn-based materials have been more or less mentioned in some systematic reviews on cathodes or polyanionic compounds essentially featuring Fe-based materials, to our knowledge no targeted reviews have reported yet, not to mention many new developments after these reports. As such, an overview of Mn-based polyanionic cathodes is timely and helpful to understand their benefits and drawbacks from a macroscopic perspective, thereby further promoting their rapid development toward applications.
2 WHERE THE ADVANTAGE LIES
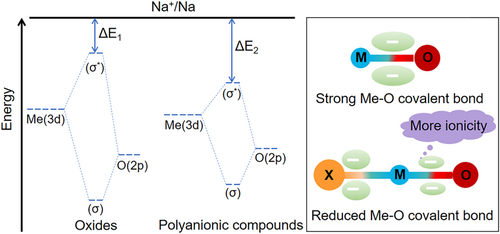
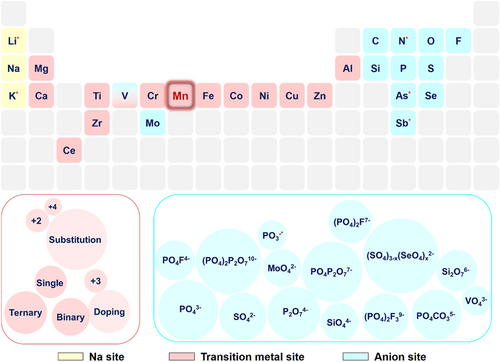
The investigation of polyanionic sodium storage cathodes can be subdivided into separate design levels for polyanionic units and charge compensation bodies. For polyanionic units, the existing ones include phosphate, pyrophosphate, sulfate, molybdate, silicate, mixed polyanions, and so on. Polyanionic compounds generally consist of tetrahedral anion (XO4)n− or its derivatives (XmO3m+1)n− (X = C, S, P, Si, Mo, etc.) and MeOx polyhedral (Me denotes transition metal) units[14], where the presence of the polyanionic units not only ensures the rapid conduction of alkali metal ions in the framework structure but also maintains the stability of the structure during the redox process of the charge compensation body. More importantly, the polyanionic compounds exhibit higher redox voltages than the oxides with the same charge compensation body, which is mainly related to transition metal coordination, polyhedra connectivity (share faces or edges), inductive effect, and d-orbital splitting (pairing energy). It has been shown that the weaker the covalent character of the Me–O bond is, the smaller the quantum mechanical repulsion between the bonding and antibonding orbitals will be, reducing the antibonding orbitals away from the Fermi energy level and thereby increasing the voltage (Figure 2). In general, the higher the coordination number of Me is, the stronger the electronegativity of the polyanionic unit will be, and then the weaker the covalency of the Me–O bond[15-19] is. As a result, the voltage characteristics of the polyanionic compounds present a certain regularity, that is, silicate < phosphate < pyrophosphate < sulfate.
For the charge compensation bodies, seven elements from Ti to Ni in the periodic table are the featured representatives that have been reported so far. Nevertheless, Fe and Mn are the only potential candidates that can be included in the research scope for environmental and price reasons alone. A typical Na4Fe3(PO4)2P2O7, for example, has the highest theoretical capacity apart from NaFePO4, reaching 129 mAh g−1, and is capable of charge compensation via Fe3+/2+ redox pair during the electrochemical process with an average voltage of around 3.2 V[20]. It can be concluded that even if the maximum capacity release can be achieved, it will be shackled to a low intrinsic operating voltage thus losing its advantage in terms of energy density. In contrast, Mn in the same polyanionic configuration can be charge compensated by Mn3+/2+ redox pair up to about 3.9 V corresponding to a theoretical specific capacity of ~129 mAh g−1, which is the highest theoretical capacity of the easily synthesized and highly active Mn-based polyanionic compounds available, almost on par with the Fe-based ones. In addition, Mn can also undergo a more reversible and stable Mn4+/3+ redox pair reaction than the Fe4+/3+ pair[21, 22]. If activated successfully, Na4Mn3(PO4)2P2O7 can theoretically provide 4 Na+ corresponding to a capacity of 172.7 mAh g−1. Therefore, the theoretical energy densities of Mn-based polyanionic cathodes are superior to that of Fe-based ones, and even if the capacity release is insufficient, their final energy densities are considerable owing to the higher voltage. At the theoretical level, Mn-based materials can possess the above-mentioned advantages; however, low actual capacity, inadequate rate capability, and poor cycling stability are also common and stubborn problems faced by these materials. By implication, it is still difficult to reach a level comparable to that of Fe-based materials in terms of these indicators. Their general progress will be discussed in the following sections by category.
3 CURRENT STATE OF THE ART
The current Mn-based polyanionic cathodes mainly include phosphates, fluorophosphates, pyrophosphates, sulfates, silicates, mixed polyanions, and so on. They feature abundant and diverse ion diffusion channels, and one or more typical representatives can be found in each category (Figure 3). The basic electrochemical properties of some of them are shown in Table 1.
| Materials | Redox couple | Voltage window (V) | Specific capacity (mAh g−1) | Diffusion coefficients (cm2 s−1) | Methods | Ref. |
|---|---|---|---|---|---|---|
| Phosphates | ||||||
| Na0.48Mn1.22PO4 | Mn3+/2+ | 1.5–4.3 | 30 (0.1 C) | [23] | ||
| NaMn0.48Mg0.02Fe0.5PO4 | Fe3+/2+, Mn3+/2+ | 2–4.3 | 122.5 (0.1 C) | [24] | ||
| NaMn0.2Fe0.8PO4 | Fe3+/2+, Mn3+/2+ | 1.5–4.5 | 134 (0.1 C) | [25] | ||
| Na4MnV0.8Al0.2(PO4)3 | Mn3+/2+, V4+/3+ | 2.5–3.8 | 94.5 (0.2 C) | ~10−10 | GITT | [26] |
| Na4MnV(PO4)3 | Mn3+/2+, V5+/4+/3+ | 1.5–4.5 | 140 (0.05 C) | [27] | ||
| Na4MnV(PO4)3 | Mn3+/2+, V4+/3+ | 2.5–3.8 | 109.9 (0.2 C) | 10−9–10−12 | GITT | [28] |
| Na4MnCr(PO4)3 | Mn4+/3+/2+ | 1.5–4.3 | 108.4 (0.1 C) | 10−8–10−13 | GITT | [29] |
| Na4MnCr(PO4)3 | Mn4+/3+/2+, Cr4+/3+ | 1.4–4.6 | 160.5 (0.05 C) | 10−11–10−12 | GITT | [30] |
| Na4MnAl(PO4)3 | Mn4+/3+/2+ | 1.5–4.3 | 90 (0.1 C) | 10−10–10−15 | GITT | [31] |
| Na4Mn0.9Mg0.1Cr(PO4)3 | Mn4+/3+/2+, Cr4+/3+ | 1.4–4.6 | 154.6 (0.1C) | 10−11–10−12 | GITT | [32] |
| Na4Mn0.9Cu0.1V(PO4)3 | Mn3+/2+, V4+/3+ | 2.4–3.8 | 117 (0.25 C) | 10−8–10−9 | GITT | [33] |
| Na4Mn0.9Ce0.1V(PO4)3 | Mn3+/2+, V4+/3+ | 2.5–3.8 | 121.2 (0.2 C) | 10−10–10−11 | GITT | [34] |
| Na4Mn0.5Fe0.5V(PO4)3 | Mn3+/2+, V5+/4+/3+, Fe3+/2+ | 2–4.0 | 120 (0.5 C) | 10−10–10−12 | GITT | [35] |
| Na4Mn0.5Fe0.5Al(PO4)3 | Fe3+/2+, Mn4+/3+/2+ | 1.5–4.3 | 102 (0.1 C) | 10−8–10−12 | GITT | [36] |
| Na3V1.99(Mg0.5Mn0.5)0.01(PO4)3 | V4+/3+ | 2.4–4.3 | 107.7 (1 C) | [37] | ||
| Na3V1.8Mn0.2(PO4)3 | Mn3+/2+, V4+/3+ | 2.5–4 | 92 (10 C) | [38] | ||
| Na3V1.875Mn0.025(PO4)3 | V5+/4+/3+ | 2.5–4 | 96.8 (0.5 C) | 9.1 × 10−13 | EIS | [39] |
| Na3MnZr(PO4)3 | Mn4+/3+/2+ | 2.5–4.3 | 105 (0.1 C) | [40] | ||
| Na3MnTi(PO4)3 | Mn4+/3+/2+ | 2.5–4.2 | 114 (0.2 C) | 9.94 × 10−10 | EIS | [41] |
| Na3MnTi(PO4)3 | Mn4+/3+/2+, Ti4+/3+ | 1.5–4.3 | 170 (0.1 C) | 2.44 × 10−10 | CV | [42] |
| Na3Mn0.95V0.05Ti(PO4)3 | Mn4+/3+/2+, Ti4+/3+ | 1.5–4.3 | 133.6 (0.1 C) | 1.02 × 10−11 | EIS | [43] |
| Na3Mn0.90Cr0.10Ti(PO4)3 | Mn4+/3+/2+, Ti4+/3+ | 1.5–4.3 | 119.6 (0.2 C) | 10−11–10−12 | CV | [44] |
| Na3Co0.5Mn0.5Ti(PO4)3 | Mn3+/2+, Co3+/2+ | 2.8–4.2 | 50 (0.1 C) | [45] | ||
| Na3.9MnCr0.9Zr0.1(PO4)3 | Mn4+/3+/2+, Cr4+/3+ | 1.5–4.5 | 156.4 (0.1 C) | 10−10–10−11 | GITT | [46] |
| Na3.75VMn0.75Al0.25(PO4)3 | Mn3+/2+, V5+/4+/3+ | 2.75–4.2 | 89 (0.1 C) | [47] | ||
| Na3.5Mn0.5V1.5(PO4)3 | Mn3+/2+, V4+/3+ | 2.5–3.8 | 113.1 (0.5 C) | 10−10–10−11 | GITT | [48] |
| Na3.2Mn0.2Cr0.8V(PO4)3 | Mn3+/2+, V5+/4+/3+ | 2–4.6 | 65.3 (5 C) | ~10−11 | EIS | [49] |
| Na3.25V1.75Mn0.25(PO4)3 | V5+/4+/3+ | 2.75–4.2 | [50] | |||
| Na2VMn2(PO4)3 | Mn3+/2+, V4+/3+/2+ | 1.5–4.5 | 80.4 (5 mA g−1) | 1.48 × 10−13 | EIS | [51] |
| Na2V0.67Mn0.33Ti(PO4)3 | Mn3+/2+, V4+/3+/2+, Ti4+/3+ | 1.5–3.8 | 110 (0.1 C) | 10−10–10−12 | CV | [52] |
| Na2Mn2V(PO4)3 | Mn3+/2+, V4+/3+ | 1.5–4.3 | 97 (0.01 A g−1) | [53] | ||
| Na2Fe2Mn(PO4)3 | Fe3+/2+ | 1.5–4 | 95 (5 mA g−1) | [54] | ||
| Fluorophosphates | ||||||
| Na3.85□0.15MnV(PO3.95F0.05)3 | Mn3+/2+, V4+/3+ | 2.5–3.8 | 108 (0.1 C) | 10−8–10−11 | GITT | [55] |
| Na3V1.95Mn0.05O2(PO4)2F | V4+/3+ | 2–4.2 | 118 (0.1 C) | 7.51 × 10−13 | EIS | [56] |
| Na3V1.95Mn0.05(PO4)2F3 | V4+/3+ | 2.5–4.3 | 122.9 (0.2 C) | 2.93 × 10−13 | EIS | [57] |
| Na3.6V1.4Mn0.6(PO4)2F3 | Mn3+/2+, V5+/4+/3+ | 2.5–4.6 | 108 (0.1 C) | [58] | ||
| Na2MnPO4F | Mn3+/2+ | 1.5–4.5 | 102.4 (0.05 C) | 2.23 × 10−16 | EIS | [59] |
| Na2Fe0.6Mn0.4PO4F | Fe3+/2+, Mn3+/2+ | 1.4–4.5 | 94.8 (0.05 C) | 1.13 × 10−15 | EIS | [60] |
| Pyrophosphates | ||||||
| Na2Fe0.5Mn0.5P2O7 | Fe3+/2+, Mn3+/2+ | 2–4.5 | 80 (0.05 C) | [61] | ||
| Na2MnP2O7 | Mn3+/2+ | 1.5–4.5 | 93 (10 mA g−1) | 10−13–10−16 | GITT | [62] |
| Na3.12Mn2.44(P2O7)2 | Mn3+/2+ | 1.5–4.5 | 114 (0.1 C) | 10−11–10−12 | CV | [63] |
| Na2MnFe2(P2O7)2 | Fe3+/2+, Mn3+/2+ | 1.5–4.5 | 86.8 (5 mA g−1) | 2.54 × 10−17 | EIS | [64] |
| Sulfates | ||||||
| Na2FeMn(SO4)3 | Fe3+/2+ | 2–4.5 | 110 (0.1 C) | 10−12–10−14 | EIS | [65] |
| Na2.5(Fe0.75Mn0.25)1.75(SO4)3 | Fe3+/2+ | 2.5–4.4 | 80 (0.05 C) | [66] | ||
| Silicates | ||||||
| Na2MnSiO4 | Mn4+/3+/2+ | 2–4.3 | 210 (0.1 C) | [67] | ||
| Na2MnSiO4 | ~10−10 | DFT | [68] | |||
| NaLiMnSiO4 | 10−9–10−16 | DFT | [68] | |||
| Na2Mn2Si2O7 | Mn4+/3+/2+ | 10−9–10−17 | DFT | [69] | ||
| Mixed polyanions | ||||||
| Na4MnFe2(PO4)2P2O7 | Fe3+/2+, Mn3+/2+ | 1.7–4.5 | 101 (2 C) | [70] | ||
| Na4Mn3(PO4)2P2O7 | Mn3+/2+ | 2–4.3 | 109 (0.05 C) | [71] | ||
| Na4Mn2Co(PO4)2P2O7 | Mn3+/2+, Co3+/2+ | 2.3–4.7 | 96.1 (0.1 C) | 5.97 × 10−14 | EIS | [72] |
| Na4Mn2.4Co0.6(PO4)2P2O7 | Mn3+/2+, Co3+/2+ | 2–4.5 | 76.8 (0.1 C) | [73] | ||
| Na4Mn0.3Fe2.7(PO4)2P2O7 | Fe3+/2+, Mn3+/2+ | 1.7–4.3 | 131.5 (0.1 C) | 10−11–10−12 | CV | [74] |
| Na4Mn0.3Co2.4Ni0.3(PO4)2P2O7 | Mn4+/3+/2+, Co3+/2+, Ni3+/2+ | 3–5.1 | 104 (0.2 C) | [75] | ||
| Na3Mn2PO4P2O7 | Mn3+/2+ | 1.8–4.3 | 101 (0.1 C) | 8.15 × 10−12 | EIS | [76] |
| Na3Mn1.6Fe0.4PO4P2O7 | Fe3+/2+, Mn3+/2+ | 1.8–4.3 | 80.7 (0.1 C) | 3.6 × 10−14 | EIS | [77] |
| Na3FeMnPO4P2O7 | Fe3+/2+, Mn3+/2+ | 1.5–4.4 | 105 (0.1 C) | 10−9–10−12 | GITT | [78] |
| Na3MnPO4CO3 | Mn4+/2+ | 2–4.5 | ~160 (~0.03 C) | 10−12–10−14 | DFT | [79] |
| Other polyanions | ||||||
| Na2.67Mn1.67(MoO4)3 | Mn3+/2+, Mo6+/5+ | 1.5–4.3 | 92 (0.1 C) | [80] | ||
| Na2Mn2.8Al0.2(VO4)3 | Mn3+/2+ | 1.5–4 | 75 (0.05 C) | [81] |
- Abbreviations: CV, cyclic voltammetry; DFT, density functional theory; EIS, electrochemical impedance spectroscopy; GITT, galvanostatic intermittent titration technique.
3.1 Phosphates
Phosphates are by far the most studied class of Mn-based polyanionic cathodes, and NaMnPO4 is one of the earliest. Unlike LiMnPO4, two polymorphs of this material exist, namely, olivine and maricite. The former is a thermodynamically substable and electrochemically active phase, while the latter is a thermodynamically stable and electrochemically inert phase[82, 83]. Specifically, Na+ and Mn2+ occupy the Mn1 (4a) and Mn2 (4c) positions, respectively, in the olivine structure, while the opposite is true in maricite. Previous theoretical calculations[84] have shown that doping of As, N, and Sb at the P-site in olivine-type NaMnPO4 reduces the band gap and the ionic character of Na–O bond, thereby achieving a simultaneous increase in electronic and ionic conductivity, with the best effect being achieved by doping Sb. NaMnPO4 prefers to form an electrochemically inert maricite phase lacking ion diffusion paths under conventional synthesis conditions. To obtain highly active materials, complex electrochemical transformations, chemical substitutions, and so on are more effective[85-88]. A detailed study of the structural stability and ion transport properties of olivine and maricite NaMPO4 (M = V–Ni) using atomic simulation techniques carried out by Zhu et al[89] is a report with reference value. The breakthrough study shows that the nanoscale maricite NaMPO4 converts to the amorphous phase MnPO4 during the electrochemical process, thus exhibiting sodium storage reversibility. Moreover, replacing part of Mn with Fe is a development direction for this material[25, 90, 91].
Mn-based phosphates based on NASICON-type Na3V2(PO4)3 derivatives are one of the current research hotspots. The highly open skeleton structure of NASICON provides three-dimensional diffusion channels and a large migration gap for ions resulting in a diffusion coefficient of sodium ions in such materials of up to 10−8 cm2 s−1. The design idea is substitution and the basic principle is electroneutrality. In the extreme case, two Mn3+ are used to completely replace V3+ to form Na3Mn2(PO4)3. Theoretical calculations suggest that it can reach an average voltage of 3.64 V, but no relevant experimental electrochemical data have been reported[92]. Comparatively, phosphates based on Mn2+ are the mainstay of research, and the design ideas of the other polyanionic compounds mentioned later are similar. One typical representative is Na3MnTi(PO4)3[41, 42, 93-96]. There are two pairs of oxidation/reduction peaks located at 3.73/3.43 and 4.14/3.94 V (Figure 4a) between a voltage window of 2.5 and 4.2 V corresponding to Mn3+/2+ and Mn4+/3+ redox couples, respectively[41]. At 0.2 C, it can deliver a capacity of 114 mAh g−1 and in situ X-ray powder diffraction (XRD) analysis shows its sodium storage involving both solid solution and two-phase reactions (Figure 4b).
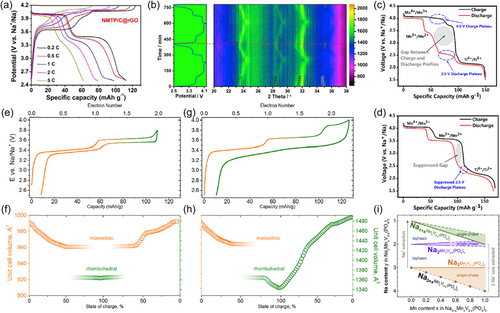
Further lowering the discharge cutoff voltage to 1.5 V activates Ti4+/3+ redox couple, allowing a further increase in capacity of ~170 mAh g−1 at 0.1 C[42, 96]. In addition, Na3MnZr(PO4)3 generated by the complete substitution of Ti4+ with Zr4+ has similar electrochemical properties[40]. Interestingly, the presence of Na+/Mn2+ cation mixing in Na3MnTi(PO4)3 leads to a significant voltage hysteresis and significantly reduces the capacity contributed by the Mn3+/Mn2+ redox couple, which can be alleviated by adding an excess of sodium during synthesis process to return the position occupied by Mn2+ to Na+ (Figure 4c,d)[95].
Another typical representative is Na4MnV(PO4)3 with a theoretical capacity of 110 mAh g−1 corresponding to two redox pairs of V4+/3+ and Mn3+/2+[27, 28, 99-103]. As already reported[97], the extraction and insertion of sodium in Na4MnV(PO4)3 proceeds via two steps between a voltage window of 2.5–3.8 V, with one sloping and one flat plateau at ~3.4 and 3.6 V. Cycling within this potential range is carried out by solid solution and two-phase processes (Figure 4e,f). If the cutoff voltage is broadened, an additional voltage plateau at ~3.9 V is observed, being associated with the unlocking of the Na1 site in the rhombohedral phase. Meantime, the reverse insertion of sodium ions proceeds through the entire solid-solution region (Figure 4g,h). This means that activation of more electron reactions (e.g., V5+/4+) can yield higher capacity and energy density, but often leads to irreversible structural evolution and changes in the storage mechanism[103]. In addition, it is worth mentioning that the sodium storage mechanism is also related to the V/Mn ratio in Na3+xMnxV2−x(PO4)3 (Figure 4i)[98, 104].
Further complete substitution of V3+ with other covalent transition metal ions can be further derived from Na4MnCr(PO4)3[29] and Na4MnAl(PO4)3[31, 105]. In these interesting systems, Mn4/3+ redox couple is easily activated below 4.3 V. As with the activation of V5/4+ redox couple in other V-based polyanions[106], Cr3+ and Al3+ can stabilize the structure like pillars, and Cr3+ can also contribute to the capacity via Cr4/3+ redox couple above 4.6 V[30, 107]. On the basis of these materials, doping or substitution of covalent or heterovalent ions such as Cu2+[33], Ca2+[108], Fe2+[109, 110], Co2+[45], Mg2+[32, 37], Al3+[26], Ce3+[34], Cr3+[44], V3+[48, 98], Zr4+[46], and so on, has also been extensively explored, some of which have beneficial effects in activating high-valent redox pairs or enhancing structural stability. Overall, these designs greatly expand the selection of Mn-based polyanionic cathodes and overcome the potential resource shortage and environmental pollution of V, providing new opportunities for large-scale applications in SIBs.
3.2 Fluorophosphates
The introduction of fluorine with greater electronegativity to enhance the inductive effect of anionic groups to obtain higher electrochemical potentials is one of the important research directions, which is mostly seen in V-based polyanionic materials[106], such as Na3V2(PO4)2F3, NaVPO4F, and Na3V2O2(PO4)2F. The current Mn-based fluorophosphate cathodes mainly include Na2MnPO4F and its partially Fe-substituted derivatives[59, 60, 68, 111-113]. Unlike Na2FePO4F with a two-dimensional layered structure, Na2MnPO4F presents a three-dimensional channel structure, whereas its ion diffusion coefficient is still as low as 10−16 cm2 s−1. At the theoretical level, Na2MnPO4F can achieve a two-electron reaction to reach a capacity of ~250 mAh g−1, corresponding to two operating voltages of about ~3.66 and ~4.67 V. Optimizing the synthesis temperature can increase the capacity from less than 50 mAh g−1 at 750°C to greater than 100 mAh g−1 at 650°C accompanied by Mn3+/2+ redox couple (Figure 5a). Comparatively, its rate performance is poor, only 26.7 mAh g−1 at 1 C[59], which may be related to the sluggish conductivity and severe Jahn–Teller effect[68].
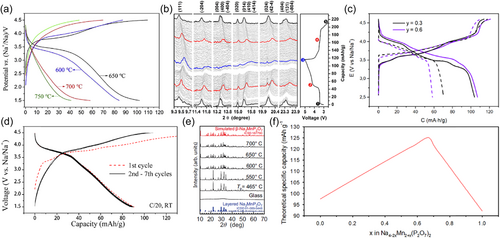
At the expense of the average voltage, the electrochemical activity can be gradually enhanced by Fe substitution. For instance, when 0.5 Mn2+ is replaced by Fe2+, the resulting Na2Fe0.5Mn0.5PO4F can deliver a capacity of 107 mAh g−1 at 0.1 C with two distinct voltage plateaus at 2.9 and 3.5 V associated with Fe3+/2+ and Mn3+/2+ redox reactions sequentially[68]. A capacity of around 70 mAh g−1 can still be obtained at 1 C but almost originates from the contribution of the Fe3+/2+ redox couple, further indicating the impoverished reaction kinetics of the Mn3+/2+ redox couple. In situ XRD analysis showed that similar to Na2MnPO4F, Na2Fe0.5Mn0.5PO4F does not display new peaks throughout the charging and discharging process (Figure 5b), indicating that a single-phase solid solution reaction mechanism is followed, which differs from Na2–xFePO4F system that forms intermediates.
In addition, Mn can also be seen in V-based fluorophosphates, and studies have shown that it demonstrates decent effects on capacity, cycling, and so on. At trace substitution (doping, <0.1), like Na3V1.95Mn0.05O2(PO4)2F[56] and Na3V1.95Mn0.05(PO4)2F3[57], Mn2+ does not change the charge/discharge characteristics of the parent material, while its effect cannot be underestimated when the substitution is larger. Instead of the galvanostatic profiles with two pronounced plateaus observed in Na3V2(PO4)2F3, the Mn-substituted samples, such as Na3.6V1.4Mn0.6(PO4)2F3 and Na3.4V1.7Mn0.3(PO4)2F3 (Figure 5c), exhibit sloping profiles consisting of several pseudoplateaus[58]. An invisible benefit is perhaps to improve the ease of battery management based on V-based phosphate or fluorophosphate cathodes.
3.3 Pyrophosphates
Since the electronegativity of pyrophosphate is slightly higher than that of phosphate, pyrophosphate cathodes exhibit higher redox potentials. Such materials can be represented by the general formula Na4−2xM2+x(P2O7)2. When M = Mn and x = 0, a typical Na2MnP2O7 is formed[62, 114-118]. The current research is dominated by the triclinic-type β-phase with three-dimensional ion diffusion channels[117]. Unlike most Mn-based cathode materials that suffer severely from sluggish kinetics, this material abnormally exhibits a capacity of up to about 90 mAh g−1 at 0.05 C with an average voltage of ~3.8 V when used as a cathode accompanied by Mn3+/2+ redox couple (Figure 5d). Moreover, it also shows remarkable cycling and rate performance, that is, 96% capacity retention after 30 cycles and 70% capacity retention at a c-rate increase from 0.05 to 1 C. This significantly enhanced kinetics is mainly ascribed to the locally flexible accommodation of Jahn–Teller distortions aided by the corner-sharing crystal structure. During charging, sodium ions are extracted first from half of the Na1 sites, immediately followed by all of the Na3 sites, and finally from half of the Na4 sites. That is, the occupancy of sodium in the four crystallographic sodium sites (Na1–Na4) is 0.5, 1, 0, and 0.5, respectively[114].
In addition to the β-phase, Na2MnP2O7 also exists in a layered phase considered to possess superior electronic conductivity and can be irreversibly transformed to the β-phase when heated to 600°C (Figure 5e). Although its electrochemical properties are still lacking (~20 mAh g−1 at 0.1 C), such concerns may be improved by optimization of grain size or carbon coating processes[115]. Using the Na2MnP2O7 as the parent, partial substitutions of Fe and Co have also been studied for sodium storage[61, 119-122].
Similar to Fe-based pyrophosphates, Mn-based pyrophosphates also exist in a series of off-stoichiometric structures, and only Na3.12Mn2.44(P2O7)2[63] and Na3.12Mn1.22Fe1.22(P2O7)2[123] have been reported so far. In Na3.12Mn2.44(P2O7)2, three pairs of redox peaks (located at 3.42/3.25, 3.81/3.63, and 4.00/3.80 V) can be identified. This off-stoichiometric structure follows the solid solution reaction mechanism and has two one-dimensional ion diffusion channels, which exhibit ion diffusion coefficients of up to 10−11 S cm−2[63], probably due to the increased carrier concentration. Following our previous experience[124-126], it seems that the theoretical capacity of such materials can also be maximized by modulating the Na/Mn ratio under the premise of electroneutrality (Figure 5f). Whether the rate capability or stability can still be reconciled remains to be supported by more experimental data.
3.4 Sulfates
According to the Pauling electronegativity rule, the bonding of S–O is stronger than that of P–O. It means that the inductive effect of sulfate is stronger than those of phosphate and pyrophosphate, and therefore the energy level resulting from the hybridization of transition metal d orbitals with O–2p orbitals in sulfates will undergo greater cleavage, thus showing higher redox potential. From the present status, there are few experimental reports on Mn-based sulfates, and only two systems, Na2FeMn(SO4)3[65] and Na2.5(Fe1−yMny)1.75(SO4)3[66], have been evaluated exactly for sodium storage performance. Such sulfates with alluaudite structure can be represented by the general formula AA′BM2(SO4)3 (M = Fe, Mn, Ni, Co), in which the sodium ions are in three different crystallographic positions, which are partially occupied A and A′ and fully occupied B, while the M sites are filled by transition metals. The presence of characteristically open channels along the [001] crystallographic directions in the formed three-dimensional network makes it possible to achieve diffusion of sodium ions at low activation energies. It was shown that Na2FeMn(SO4)3 can deliver a capacity close to 110 mAh g−1 at 0.1 C with an average voltage of 3.6 V and is generally between FeFe and FeNi analogs (Figure 6a). In a series of off-stoichiometric Na2.5(Fe1−yMny)1.75(SO4)3 solid solutions, the average operating voltage gradually increases with increasing Mn substitution, yet it is accompanied by a rapid capacity decay, that is, from 110 mAh g−1 without Mn to about 0 mAh g−1 with Mn only (Figure 6b), which is mainly attributed to the entire Mn2+ inactivity during cycling and charge compensation originates only from Fe3+/2+ redox couple.
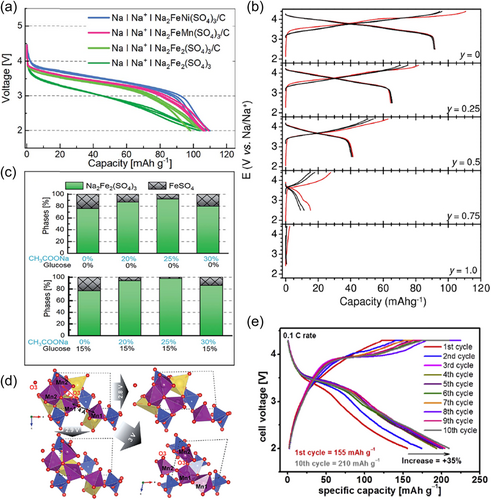
As for other materials such as Na2Mn3(SO4)4[128, 129], NaxFe0.5Mn0.5(SO4)2[130], Na2+dMn2−d/2(SO4)3[131, 132], Na2.44Mn1.79(SO4)3[133], Na2Mn(SO4)2·nH2O[134], K0.5Na1.5Mn1.5Mg0.5(SO4)3[135], and so on, they are still only available at the level of theoretical studies or experimental synthesis. Generally, sulfates can be synthesized below 450°C by low-temperature solid-state, ionothermal or solution processes. However, what is often obtained are some off-stoichiometric phases containing impurities, so it is urgent to improve their crystallinity and purity in addition to addressing thermal stability. A more successful experience is the addition of excess sodium or organic carbon sources during the synthesis, for example, when 25 mol% of CH3COONa together with 15 mol% of glucose was used, the amount of Na2Fe2(SO4)3 in the final product can be significantly increased from 76 to 98 wt% without additional additive (Figure 6c).
3.5 Silicates
Silicate polyanionic materials having the advantages of low cost and high elemental abundance play a delightful role in lithium-ion batteries (LIBs). In SIBs, two types of Mn-based silicates have been reported, one is Na2Mn2Si2O7[69, 127]. Unlike edge-sharing connectivity of Mn–O polyhedra in Li2Mn2Si2O7, corner-sharing in Na2Mn2Si2O7 is apparent, and this structure theoretically enables the strain introduced by the Jahn–Teller distortion of Mn3+ to be relieved by taking only smaller atomic rearrangements. It can deliver a theoretical capacity of 165 mAh g−1, but even after carbon coating, only about 25% is actually released with an average discharge voltage of only 2.2 V[127]. Such sluggish kinetics may be related to the rearrangement of the Mn coordination accompanied by a substantial volume contraction (Figure 6d)[127] or possibly to the huge migration coefficient change of the sodium ions during cycling[69].
The other one is Na2MnSiO4 with three-dimensional ion diffusion channels[67, 136-139], which has a much higher diffusion coefficient than the lithium analog due to a larger spacing between atomic layers and to the nondirectional bonding between sodium and the neighboring atoms[138, 139]. Moreover, the doping of other ions such as Al3+ at the Si site is expected to further optimize its ion diffusion properties[137]. It was shown that by adding 5% VC to a conventional organic liquid electrolyte, the capacity of Na2MnSiO4 can be significantly increased from 129 to 155 mAh g−1 in the first cycle and further to 210 mAh g−1 after 10 cycles at 0.1 C (Figure 6e)[67]. Such a high capacity contribution is attributed to the two-electron reaction and the structural stability of the active material itself (no amorphization like Li2MnSiO4) as well as the overall electrode during charge and discharge.
3.6 Mixed polyanions
Such electrode materials usually contain two or more polyanionic units, and the pairing of different anion groups can broaden the structural and redox potential diversity. For Mn-based materials, Na3MnPO4CO3[79, 140-146], Na4Mn3−xMx(PO4)2P2O7 (M is one or more of Fe, Co and Ni, 0 ≤ x < 3)[70-75, 147-149], Na3Mn2−xFexPO4P2O7 (0 ≤ x < 2)[76-78, 150], and Na2+2zMn2−z(SO4)3−x(SeO4)x[151] have been reported so far.
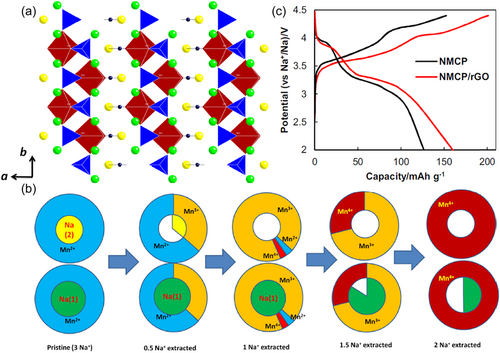
Na3MnPO4CO3 belongs to the category of sidorenkite that have rarely been explored and was used as a sodium storage cathode until 2013 by Ceder's group[146]. It has a peculiar structure in which the MnO6 octahedra are connected by PO4 tetrahedra to form bilayers, while the CO3 groups share an oxygen edge with the MnO6 octahedra and point toward a bilayer. The sodium atoms are located in two different interstitial sites, namely, Na1 and Na2 (Figure 7a), and theoretical studies[145] have shown that these sodium ions can diffuse in three directions with low activation energy barriers. During the electrochemical process, the sodium ions at the Na2 site are extracted first, and then 1/2 of the sodium ions at the Na1 site are extracted (Figure 7b), along with a two-electron reaction resulting in a theoretical capacity of up to 191 mAh g−1, which is rare among the mixed polyanions. The current better-optimized sample, graphene-based composite, can achieve a capacity of 160 mAh g−1 at 0.01 C between the voltage limits of 2.0 and 4.4 V accompanied by two plateaus at 3.9 and 3.3 V that can be attributed to Mn4+/3+ and Mn3+/2+ redox couples, respectively (Figure 7c)[144]. The main problem of this material is that the electronic conductivity is so poor that its rate capability is not desirable. To stimulate its potential, excessive carbonaceous additions once became necessary[141]. Moreover, the sluggish kinetics may also be related to the crystallinity of the sample, after all, its synthesis is usually employed by low-temperature hydrothermal or ball milling methods.
Na4Mn3(PO4)2P2O7 can be obtained when x = 0 in Na4Mn3−xMx(PO4)2P2O7 (M is one or more of Fe, Co, and Ni, 0 ≤ x < 3). This material with a three-dimensional open structure containing four crystallographic sodium sites is currently one of the most deserving of attention among the mixed polyanions. Upon charging, the Na2 site preferentially extracts sodium ions and follows a single-phase reaction (below ~3.51 V) until one sodium ion is completely extracted, then immediately transfers to a two-phase reaction process and extracts another 0.25, 0.25, and 0.5 sodium at the Na1, Na3, and Na4 sites, respectively (Figure 8a). The state of the art dates back to 6 years ago, that is, a capacity of 80 mAh g−1 at 5 C accompanied with Mn3+/2+ redox coupled with an average voltage of 3.8 V (Figure 8b). After 500 cycles, its capacity has almost no decay[71]. It is common knowledge that the Mn3+ suffers from a severe Jahn–Teller effect, which is often one of the main factors that are detrimental to the electrochemical performance. However, this effect is rather anomalous in this material, meaning that the distortion of the MnO6 octahedral instead promotes its electrochemical kinetic behavior. If the Mn2+ is partially substituted with Fe2+, Co2+, or Ni2+ to form binary or ternary phases, the electrochemical properties such as voltage, capacity, and cycle life will be modulated[70, 72-75].
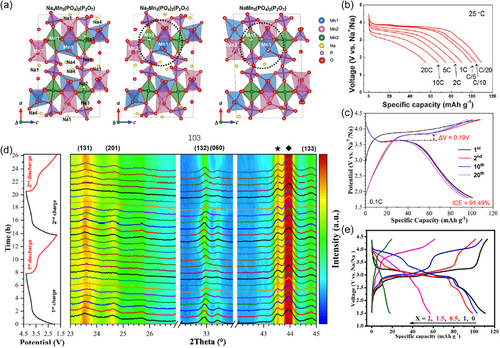
Na3Mn2PO4P2O7 is a new paradigm recently reported with a theoretical capacity of 119 mAh g−1 slightly lower than that of Na4Mn3(PO4)2P2O7. It can deliver a capacity of 101 mAh g−1 at 0.1 C with an average operating voltage of 3.6 V, corresponding to the redox reaction of the Mn3+/2+ redox couple (Figure 8c). Further studies show that the material contains three crystallographic sodium sites, where sodium ions in the Na1 and Na3 sites are more easily extracted during charge, and follows only the solid-solution reaction mechanism throughout the cycle (Figure 8d)[76]. It is worth mentioning that the volume change of the material is as low as 0.87% during desodiation/sodiation, which can essentially be considered as a zero-strain material. Partial substitution of Mn2+ with Fe2+ is beneficial to yield a highly active material but sacrifices part of the voltage strength. Optimally, acceptable results can be obtained when the Mn/Fe ratio is 1:1 (Figure 8e)[78]. Moreover, these resulting binary phases follow the same single-phase reaction mechanism as Na3Mn2PO4P2O7[78, 150].
For Na2+2zMn2−z(SO4)3−x(SeO4)x using monoclinic C2/c alluaudite-type structure, it can be regarded as a derivative obtained by partial substitution of SO42− in Na2Mn2(SO4)3 with SeO42−. As the selenate content increase, the electrochemical activity of such mixed alluaudites gradually increases accompanied by a decrease in the average voltage (Figure 9a), in agreement with the lower electronegativity of Se compared to that of S. Only a minor part of the total electrochemical activity could be attributed to Mn3+/2+ redox couple and the additional capacity may originate from the Se6+/4+ couple (~2.25 V)[151]. Studies on the sodium storage behavior of such materials are relatively poor, and the only data available (60 mAh g−1 at 0.05 C) do not show any competitive advantage in terms of electrochemical performance compared to other polyanions.
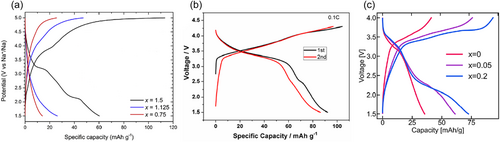
3.7 Other polyanions
Apart from the above-mentioned categories, there are some special Mn-based polyanionic cathodes worth mentioning. For example, Na2.67Mn1.67(MoO4)3, which belongs to the alluaudite family, sodium ions can migrate along c-axis and b-axis in its three-dimensional framework structure, while in Na2.76Mn1.78(SO4)3, sodium ions can only migrate along the c-axis, implying a higher mobility. When used as a cathode, it can liberate a capacity of 92 mAh g−1 at 0.1 C with an average discharge voltage of about 3.45 V (Figure 9b)[80]. It is demonstrated that the capacity contribution mainly originates from the Mn3+/2+ redox couple (~3.5 V), while a small portion comes from the Mo6+/5+ redox couple (~2.4 V), for which further verification is needed. Following Na2Mn2Fe(VO4)3 with low capacity, a new alluaudite, Na2Mn3−xAlx(VO4)3 (x = 0, 0.05, and 0.2), has recently emerged. With increasing Al3+ content, the electrochemical activity of the as-prepared material at 0.05 C rises significantly from 35 mAh g−1 at x = 0 to 75 mAh g−1 at x = 0.2 (Figure 9c). This positive effect is attributed, on the one hand, to the greater ionicity of the V–O bond optimizing electron transport and, on the other hand, to the Al3+ alleviating the Jahn–Teller distortion. Another example is NaFe1−xMnx(PO3)3 (x = 0–1)[152], but its electrochemical details published so far are only for x = 0, that is, materials containing Mn have yet to be investigated.
4 OVERVIEWS OF OPTIMIZATION
The electrochemical activity of Mn-based cathodes is typically worse than that of Fe- or V-based cathodes. The reasons are mainly due to low electronic conductivity, large lattice mismatch, slow phase boundary mobility, large effective mass of polariton, high volume strain, strong Jahn–Teller distortion, and lack of minority of nonbonding electrons[114]. In this regard, many strategies including synthesis, composite, coating, nanosizing, doping, and substitution have been proposed and achieved good results and a brief overview will be carried out in the following.
Mn-based polyanionic cathodes are commonly obtained by solid-state[62, 111, 123, 153], sol–gel[38, 39, 43, 51, 52, 154-156], spray drying[34, 74, 112, 153], and combustion methods[33], a few of which can also be prepared by aqueous solution (e.g., sulfate)[65], hydrothermal/solventothermal (e.g., V-based fluorophosphates)[54, 58, 79, 141-143], and ball milling (e.g., sidorenkite)[140, 144]. While the larger framework structure enables the polyanionic compounds to have better ionic conductivity, the discontinuity between the metal octahedral in the crystal structure renders the electronic conductivity worse than that of the layered oxides, which proves to be almost fatal for Mn. Suffice it to say that the key challenge facing Mn-based polyanions is that materials with excellent properties are not so easy to synthesize, which may be one of the reasons for the slow progress in the research of such materials; after all, it is not good to be surrounded by negative energy all the time. To modify the electrochemical properties of Mn-based polyanions, nanostructure design is a successful attempt, such as hollow spheres, nanofibers, and hydrangeas[25, 56, 99, 150].
In general, carbon composite strategy accounts for a large part of the existing research, among which the effect of dual carbon composite is especially remarkable[101, 102, 107, 153, 157]. This strategy is to achieve further performance enhancement by introducing appropriate amounts of reduced graphene oxide or carbon nanotubes, and so on, in conjunction with disordered carbon. In terms of effectiveness, the nature of carbon is important for enhanced electrochemical properties, and there seems to be a consensus for complementary doping (e.g., N doping[94]). In addition, the choice of carbon source should be taken into account, for example, pyromellitic acid has been proven to be an efficient carbon source for the synthesis of high-performance polyanions due to the good electronic conductivity of its derivatives[70, 71, 149].
Atomic-level-based doping means have shown excellent results in suppressing the Jahn–Teller effect of high-spin Mn3+ and in enhancing the intrinsic electronic/ionic conductivity, and are often used to alleviate many issues present in layered oxide cathodes[158-161]. Similarly, doping of polyanionic compounds not only enhances electronic conductivity but also activates additional charge compensation of high-valent redox couples (e.g., V5+/4+)[162-165]. Current doping studies for Mn-based polyanions are mainly on active ion doping, which can also be regarded as a partial substitution; after all, the boundary between doping and substitution is not very clear at present. The introduction of such active components tends to change the original sodium storage behavior and is accompanied by a change in the voltage profile. It should be noted that the ion activity may also be related to the content, and its effect on the charge/discharge curve is almost imperceptible if introduced at trace levels. Although inert ion doping such as Mg2+ and Zr4+ has also been reported[32, 37, 46], it is relatively uncommon. In addition to doping substitution regarding the charge compensation body, anion doping is also one of the modification strategies, for example, the replacement of O2− in Na4MnV(PO4)3 by trace amounts of F− can yield Na3.85□0.15MnV(PO3.95F0.05)3 with a significant increase in capacity and rate performance, probably due to the incorporation of F− in the structure producing sodium deficiency, which contributes to the enhanced sodium ion diffusion and also improves the reversibility in the high voltage region[55].
5 CONCLUSIONS AND PERSPECTIVES
Cost is the core element that drives the competition between SIBs and LIBs. Mn is expected to have a place in cathode materials for sodium storage due to its advantages of environmental friendliness, low price, and high annual production. In electrode materials, it can be used as a charge compensation body or as a doping/substitution protagonist. Truth be told, polyanionic cathodes are not favored in practical applications mainly due to their decent capacity. Fortunately, the presence of Mn offers a glimmer of hope for such materials. In view of this, this paper presents a review of the research landscape of Mn-based polyanionic cathodes and provides an overview of the material optimization strategies. Overall, there is a wide variety of Mn-based polyanionic cathodes. As two typical representatives, NASICON structure is slightly better in terms of both electrochemical activity and availability, while those of pyrophosphates are not so successful.
In the framework of polyanionic compounds, the electron cloud of transition metal valence electrons is isolated, thereby hindering the exchange of electrons. Coupled with the fact that electron transfer between transition metal ions is hindered by the polyanionic groups, the electron conductivity of polyanionic cathodes is generally low. To solve the mentioned issues, a common approach in Mn-based polyanionic cathodes is to construct carbon composite or carbon-coated nanosized electrode materials. This approach has been validated in previous studies, but the morphology design is more monotonous without breakthroughs, and the carbon content is usually very high, generally between 10 and 20 wt%, which undoubtedly has a negative impact on the overall energy density of the battery when applied on a large scale. Therefore, it is urgent to design efficient modification strategies for Mn-based polyanionic cathodes based on a significant reduction of carbon content (<5 wt%), which is perhaps the only way to give polyanionic compounds a place in the arena of practical cathodes.
Doping/substitution has been uniquely effective in enhancing the overall electrochemical performance and optimizing the sodium storage mechanism. Some key information can be gleaned from analyzing the results of layered oxides: the nature of doping is to “break” the inherently ordered structure (e.g., cation, charge, and sodium vacancy ordering) to “reconfigure” its energy level structure, while disordering helps to substantially improve the intrinsic electrochemical properties of electrode materials. The disordering contributes to the intrinsic electrochemical properties of electrode materials. Specifically, cation ordering is mainly related to the radius size of transition metal cations, and the more similar the radii of the two ions are, the more they tend to be disordered, while the interplay between charge ordering and sodium vacancies is mainly related to the Fermi energy levels, or redox potentials, between the cations. The greater the difference in the Fermi energy levels of the two ions is, the more the charge and sodium vacancies tend to be arranged in a disordered manner. Thus, it can be assumed that such generalized knowledge may also be suitable to guide the “reconstruction” of Mn-based polyanions. However, not much research has been done on doping in Mn-based polyanions in a strict sense, let alone in depth. Perhaps, a systematic study to analyze in depth of the order and disorder of the microstructure under single or multiple ion synergy and to investigate the related conformational relationships is a worthy direction to be considered.
Furthermore, how to maximize the capacity release, how to enhance the cycling stability, how to enhance the rate performance, and how to activate the Mn4+/3+ redox couple for charge compensation should be of special interest. The elucidation of these fundamental issues will be the most basic guarantee to guide us to design and prepare high-performance Mn-based polyanionic cathodes on a large scale. Meanwhile, it is also essential to accelerate the research on electrolytes with wide electrochemical windows and high stability to match the new high-efficiency and low-cost polyanionic cathodes.
AUTHOR CONTRIBUTIONS
Yubin Niu: Conceptualization; investigation; writing – original draft and review and editing. Yanan Zhao: Investigation and writing – original draft and review and editing. Maowen Xu: Supervision. All authors contributed to the discussion of the results and commented on the manuscript at all stages.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 22005315), Fundamental Research Funds for the Central Universities (Grant Nos. SWU119050 and SWU120080), and Chongqing Key Laboratory of Materials Surface & Interface Science (Grant No. KFJJ2002).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Biographies

Yubin Niu is currently an associate professor at the School of Materials and Energy at Southwest University. He received his Ph.D. degree from Southwest University in 2017 and then worked as a postdoctoral researcher at the Institute of Chemistry, Chinese Academy of Sciences (ICCAS) from 2017 to 2020. His current research interests focus on electrode materials and electrolytes for advanced energy storage devices including solid batteries and metal ion batteries.

Yanan Zhao is currently a lecturer at the analytical and testing center of Southwest University. She received her Ph.D. from the Bioengineering College of Chongqing University in 2019. Her current research interests include photoelectric-sensitive functional nanostructured materials, biosensing, energy storage, and corresponding applications of advanced characterization technologies.

Maowen Xu is currently a professor at the School of Materials and Energy at Southwest University. He received his Ph.D. degree from Lan Zhou University in 2008. He joined Prof. J. B. Goodenough's group at the University of Texas at Austin and worked as a postdoctoral researcher from 2010 to 2013. He has published over 200 peer-reviewed journal papers. His current research is focused on the design and synthesis of electrode materials for sodium-ion batteries and lithium/sodium sulfur batteries.




