Characterization of multiciliated ependymal cells that emerge in the neurogenic niche of the aged zebrafish brain
Grant sponsor: The Japan Society for the Promotion of Science; Grant number: 15H01217 (to K.S.); Grant number: 26250019 (to K.S.); Grant sponsor: The Research Funding for Longevity Science from the National Center for Geriatrics and Gerontology; Grant number: 26-25 (to K.S.); Grant sponsor: Valencian local Ministry of Education; Grant number: PROMETEOII/2014/075 (to J.M.G.-V.); Grant sponsor: The Network for Cell Therapy TerCel of the Health Institute Carlos III; Grant number: ISCIII2012-RED-19-016 (to J.M.G.-V.); Grant sponsor: The Japan Society for the Promotion of Science Bilateral Open Partnership Joint Research Projects (to K.S., J.M.G.-V.); Grant sponsor: Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (to K.S., J.M.G.-V.).
ABSTRACT
In mammals, ventricular walls of the developing brain maintain a neurogenic niche, in which radial glial cells act as neural stem cells (NSCs) and generate new neurons in the embryo. In the adult brain, the neurogenic niche is maintained in the ventricular-subventricular zone (V-SVZ) of the lateral wall of lateral ventricles and the hippocampal dentate gyrus. In the neonatal V-SVZ, radial glial cells transform into astrocytic postnatal NSCs and multiciliated ependymal cells. On the other hand, in zebrafish, radial glial cells continue to cover the surface of the adult telencephalic ventricle and maintain a higher neurogenic potential in the adult brain. However, the cell composition of the neurogenic niche of the aged zebrafish brain has not been investigated. Here we show that multiciliated ependymal cells emerge in the neurogenic niche of the aged zebrafish telencephalon. These multiciliated cells appear predominantly in the dorsal part of the ventral telencephalic ventricular zone, which also contains clusters of migrating new neurons. Scanning electron microscopy and live imaging analyses indicated that these multiple cilia beat coordinately and generate constant fluid flow within the ventral telencephalic ventricle. Analysis of the cell composition by transmission electron microscopy revealed that the neurogenic niche in the aged zebrafish contains different types of cells, with ultrastructures similar to those of ependymal cells, transit-amplifying cells, and migrating new neurons in postnatal mice. These data suggest that the transformation capacity of radial glial cells is conserved but that its timing is different between fish and mice. J. Comp. Neurol. 524:2982–2992, 2016. © 2016 Wiley Periodicals, Inc.
Neurogenesis in the adult brain is widely conserved in vertebrates, from fish to humans (Garcia-Verdugo et al., 2002; Grandel and Brand, 2013; Sawada and Sawamoto, 2013; Paredes et al., 2015). Brain ventricular walls maintain a neurogenic niche, in which ventricle-contacting neural stem cells (NSCs) generate new functional neurons. In the mammalian brain, radial glial cells with a primary cilium cover the surface of the ventricular wall of lateral ventricles and act as NSCs during embryonic development (Malatesta et al., 2000; Miyata et al., 2001; Noctor et al., 2001). On the other hand, in the adult brain, the neurogenic niche is maintained in the ventricular-subventricular zone (V-SVZ) of the lateral wall of lateral ventricles (Alvarez-Buylla and Garcia-Verdugo, 2002; Kaneko and Sawamoto, 2009). Ventricular walls in the adult brain are covered with multiciliated ependymal cells and monociliated astrocytic NSCs (Doetsch et al., 1997, 1999). In the V-SVZ, astrocytic NSCs give rise to neuronal progenitor cells, which are actively proliferating cells. These cells subsequently generate migrating new neurons, which are immature neurons with features of migratory cells (Doetsch et al., 1999). These new neurons migrate tangentially through the rostral migratory stream (RMS) and differentiate into interneurons in the olfactory bulb (OB) as part of the olfactory system (Lois and Alvarez-Buylla, 1994; Doetsch and Alvarez-Buylla, 1996). Previous studies suggest that embryonic radial glial cells transform into astrocytic NSCs and ependymal cells after birth, leading to the establishment of a neurogenic niche in the adult brain (Merkle et al., 2004; Spassky et al., 2005).
Compared with mammals, zebrafish maintain a higher neurogenic potential, even in the adult brain. Monociliated radial glial cells persist in the telencephalic ventricular zone (VZ) of the adult zebrafish (Adolf et al., 2006; Grandel et al., 2006; Lam et al., 2009; Ganz et al., 2010; Marz et al., 2010; Kishimoto et al., 2011). Migrating new neurons are actively generated in the ventral telencephalic VZ, which is located along the subpallium and corresponds to the mammalian V-SVZ, and migrate rostrally toward the OB and laterally toward the telencephalon (Adolf et al., 2006; Grandel et al., 2006; Kishimoto et al., 2011). A recent study suggested that the neurogenic potential in the telencephalic VZ of zebrafish decreases with aging (Edelmann et al., 2013). However, the effects of aging on the cell composition and organization of the ventral telencephalic VZ in the zebrafish brain are unknown.
The present study uses immunocytochemistry, electron microscopy, and live imaging techniques to study the age-related changes of the ventral telencephalic VZ in zebrafish. We found that multiciliated ependymal cells, which resemble those in postnatal mice, emerge in the dorsal part of the ventral telencephalic VZ of aged fish.
MATERIALS AND METHODS
Animals
All experiments were performed with adult (3–24-month-old) zebrafish (Danio rerio). Zebrafish were maintained by using standard procedures (Westerfield, 2000). Wild-type AB (RRID:ZIRC_ZL1) and transgenic Tg(gfap:gfp)mi2001 zebrafish (RRID:ZFIN_ZDB-GENO-060623-2; Bernardos and Raymond, 2006) were obtained from the Zebrafish International Resource Center (ZIRC). To generate Tg(gfap:gfp)mi2001 zebrafish, a gfap:gfp expression vector containing 7.4 kb of gfap regulatory elements in front of the EGFP-1 gene was injected into an embryo (Bernardos and Raymond, 2006). Three- to ten-month-old fish were designated as young adult and 12–24-month-old fish as aged fish. The animal protocols used in this study were approved by the Laboratory Animal Care and Use Committee of Nagoya City University.
Immunohistochemistry and confocal microscopy
Fish were anesthetized with Tricaine as described previously (Kishimoto et al., 2011). The brain was dissected and fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer at 4°C overnight. To prepare brain sections, the whole brain was embedded in 3% agarose in phosphate-buffered saline (PBS), and 50-μm-thick floating coronal and sagittal sections were prepared using a vibrating microtome (Leica VT-1200S). The sections were incubated for 1 hour at room temperature (RT) in 0.3% Triton X-100 and 10% normal donkey serum in PBS, overnight at 4°C with the primary antibodies, and for 2 hours at RT with Alexa-Fluor-conjugated secondary antibodies (1:500; Invitrogen, Carlsbad, CA). Hoechst 33342 (1:5,000; Sigma, St. Louis, MO) was used for nuclear staining. Sections were mounted on glass slides in PermaFluor (Thermo Scientific. Fair Lawn, NJ).
The sections were scanned with a confocal laser scanning microscope (LSM700; Carl Zeiss) with a water-immersion objective lens (×40). The total number of ciliated cells in the ventral telencephalic VZ was counted by using 1-μm-interval z-stacked images. The brightness and contrast of images were modified in Adobe Photoshop (RRID:SCR_014199). The length of cilia was measured in ZEN software (Carl Zeiss; RRID:SCR_013672).
Description of the primary antibodies used
Antiacetylated α-tubulin
The acetylation of α-tubulin is an important characteristic of axoneme assembly in various organisms. The antiacetylated α-tubulin antibody (Sigma; catalog No. 6-11B-1, RRID:AB_477585) detects an epitope on the α3 isoform of Chlamydomonas axonemal α-tubulin, within four residues of Lys40 when this amino acid is acetylated. In Western blots of protein fractions containing axonemal microtubules from a variety of sources, including the cilia from sea urchin blastulae and Tetrahymena, sperm and testis from Drosophila, and human sperm, this antibody detects a single band (Piperno and Fuller, 1985). In our study, this antibody stained cilia in zebrafish brain.
Anti-S100
S100 is a multigene family of calcium-binding proteins. The anti-S100 antibody (DAKO, Carpinteria, CA; catalog No. Z0311, RRID:AB_10013383) detects S100B strongly and S100A1 and S100A6 weakly. It does not react with S100A2, S100A3, or S100A4 (Ilg et al., 1996). In immunoblots with zebrafish extracts, this antibody detects a single band (10–12 kDa; Gayoso et al., 2011). In our study, this antibody stained glial cells in the telencephalic VZ in the young-adult and aged zebrafish brain.
Transmission electron microscopy
The brain was fixed in 2% glutaraldehyde (GA) and 2% PFA in 0.1 M cacodylate buffer (pH 7.4) at 4°C, treated with 2% OsO4 in the same buffer at 4°C, dehydrated in a graded ethanol series, placed in propyleneoxide, and embedded in Quetol 812 epoxy resin for 48 hours at 60°C to ensure polymerization. Ultrathin sections (90–100 nm) were cut with an Ultracut-E (Reichert-Jung) with a diamond knife and stained with 2% uranyl acetate in distilled water for 15 minutes and with modified Sato's lead solution (Hanaichi et al., 1986) for 5 minutes. Sections were analyzed with a transmission electron microscope (JEM-1011J; JEOL, Tokyo, Japan).
Scanning electron microscopy
The brain was fixed in 2% GA in 0.1 M cacodylate buffer (pH 7.4) at 4°C and additionally treated with 2% OsO4 in the same buffer at 4°C for 2 hours. Samples were dehydrated in ethanol, dried in a critical-point dryer (CPD300; Leica), and coated with a layer of sublimated OsO4 with an osmium plasma coater (OPC80AJ; Filgen, Nagoya, Japan). Specimens were examined under a scanning electron microscope (H-4800; Hitachi, Tokyo, Japan). The number of cilia per cell was counted.
Live imaging of ciliary beating and fluid flow
To observe ciliary beating, acute brain slices (100–200 μm thick) were prepared from 12-month-old transgenic Tg(gfap:gfp)mi2001 zebrafish and cultured in Neurobasal medium containing 10% fetal bovine serum (FBS), 50 U/ml penicillin-streptomycin, 2 mM L-glutamine, and 2% B27 supplement. The RMS region was identified based on its location and by its appearance as a tube-like structure surrounded by the curved ventricular walls of both hemispheres (Fig. 1A) and its lower levels of GFP expression compared with other parts of the ventricular walls in Tg(gfap:gfp)mi2001 fish (Marz et al., 2010). Time-lapse video recordings were performed by differential interference contrast microscopy using a microscope (BX51; Olympus) with a ×40 objective lens and a high-speed, progressive-scan charge-coupled device camera (HAS-L2; DITECT; 200 frames per sec, 1,280 × 1,024 pixels). The time required for one stroke was measured, and the ciliary beat frequency was calculated. To visualize the fluid flow generated by ciliary beating, fluorescent beads (0.1-μm; Invitrogen) were deposited into the culture medium of a brain slice (final concentration 0.002%). The movement of beads was recorded at 200 frames per sec using a high-speed camera (HAS-L2; DITECT; 1,280 × 1,024 pixels) equipped with a microscope (BX51; Olympus). After imaging live tissues, the movement of beads by control samples (fixed with 4% PFA in 0.1 M phosphate buffer at RT) was recorded by using the protocol described above.
Statistical analysis
The data are presented as the mean ± SEM. Comparisons between two groups were analyzed by the unpaired t-test. Differences were regarded as statistically significant at P < 0.05.
RESULTS
Multiciliated cells emerge in the dorsal part of the ventral telencephalic ventricular zone in aged zebrafish
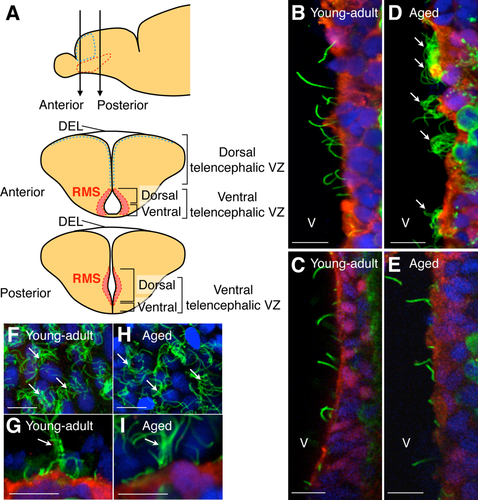
In zebrafish, monociliated radial glial cells in the telencephalic VZ are maintained even in the adult stage (Adolf et al., 2006; Grandel et al., 2006; Lam et al., 2009; Ganz et al., 2010; Marz et al., 2010; Kishimoto et al., 2011). However, the effects of aging on ciliated cells in the ventral telencephalic VZ of the zebrafish brain have not been investigated. To observe cilia on the surface of the ventral telencephalic VZ, we performed immunohistochemical analyses with brain sections prepared from young-adult and aged zebrafish (Fig. 1A). In young-adult fish (3–10 months old), the ventral telencephalic ventricular wall was covered with S100-positive (S100+) cells, almost all of which extended an acetylated tubulin+ primary cilium (98.2% ± 1.0%, 165 cells examined; Fig. 1B,C). In the aged fish (12–24 months old), the density of monociliated cells was significantly decreased (young adult 5,965 ± 428 cells/mm2, aged 2,627 ± 485 cells/mm2; P = 0.0043, t-test). We observed predominantly multiciliated S100+ cells in the dorsal part of the ventral telencephalic VZ (1,414 ± 329 cells/mm2) of the aged fish (Fig. 1D,E), where new neurons migrate toward the olfactory bulbs (Fig. 1A; Adolf et al., 2006; Kishimoto et al., 2011). In the dorsal ependymal lining (DEL) and dorsal telencephalic VZ (Fig. 1A), where multiciliated cells exist even in young-adult fish (Lindsey et al., 2012), the density of multiciliated cells was unchanged between young-adult and aged fish (young-adult DEL 9,354 ± 777 cells/mm2, aged DEL 8,887 ± 528 cells/mm2; P > 0.05, t-test; young-adult dorsal telencephalic VZ 51 ± 42 cells/mm2; aged dorsal telencephalic VZ 31 ± 27 cells/mm2; P > 0.05, t-test; Fig. 1F–I), suggesting that the emergence of multiciliated cells with aging is restricted to the dorsal part of the ventral telencephalic VZ. The ciliary length in the multiciliated cells was 8.94 ± 0.51 μm (16 cells from three fish), which is similar to the ciliary length in mouse ependymal cells (Hirota et al., 2010). These results suggested that multiciliated cells emerge along the RMS in the dorsal part of the ventral telencephalic VZ of the aged zebrafish brain.
Multiple cilia align in the dorsal part of the ventral telencephalic ventricular walls in aged zebrafish
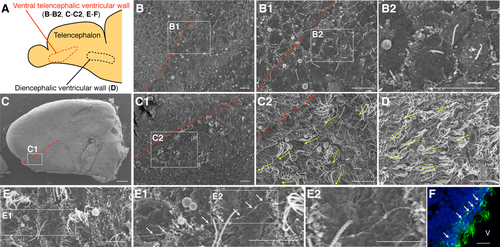
To examine the morphology of the cilia in more detail, we observed the surface of the telencephalic ventricle in young-adult and aged fish by scanning electron microscopy (Fig. 2A). In young-adult fish, only monociliated cells were observed in the ventral telencephalic ventricular walls (Fig. 2B). These primary cilia were extended in random directions (Fig. 2B1,B2). On the other hand, in aged fish, multiciliated cells, in addition to monociliated cells, could be observed in the dorsal part of the ventral telencephalic ventricular walls (Fig. 2C,C1). The number of cilia per cell was 16 ± 1 (20 cells from three fish). The multiple cilia were oriented in the rostral direction (Fig. 2C2), similar to those in the diencephalic ventricular walls (Fig. 2D). Thin fibers extending along the ventral telencephalic ventricular walls were observed in aged fish by SEM (Fig. 2E–E2) and antiacetylated tubulin staining (Fig. 2F). Taken together, these scanning electron microscopy data suggested that the cilia on the emerged multiciliated cells show a rostral orientation and align in the dorsal part of the ventral telencephalic VZ.
Multiple cilia on the aged ventricular wall beat coordinately and generate fluid flow
To reveal the function of the multiple cilia in the ventral telencephalic VZ in aged fish, we performed live imaging of the ciliary behavior in horizontal brain slices of the aged fish telencephalon. Horizontal sectioning allowed us to visualize the direction of the ciliary beating of multiciliated cells aligned along the rostrocaudal axis of the ventral telencephalic VZ. We found that these cilia beat coordinately in the rostral direction at a frequency of 9.17 ± 0.74 Hz (21 cells from three fish; Supp. Movie 1), which is comparable to the ciliary beating frequency in the diencephalic ventricular walls (9.59 ± 1.5 Hz; 19 cells from six fish; Supp. Movie 2).
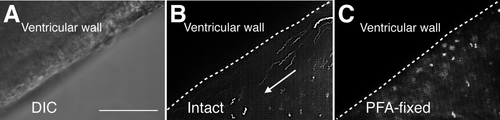
Next, to examine whether the beating of multiple cilia generates fluid flow, we deposited fluorescent beads into the culture medium of a horizontal brain slice and observed the bead movement by time-lapse video recording. Beads floating close to the ventricular surface moved in the rostral direction (Supp. Movie 3), but not in the same samples after PFA fixation (Supp. Movie 4; Fig. 3A–C). These results suggested that the coordinated beating of the multiple cilia that emerged in aged fish generates constant and directional fluid flow within the telencephalic ventricle.
The dorsal part of the ventral telencephalic VZ of aged zebrafish has cellular components similar to those of the adult mouse ventricular-subventricular zone
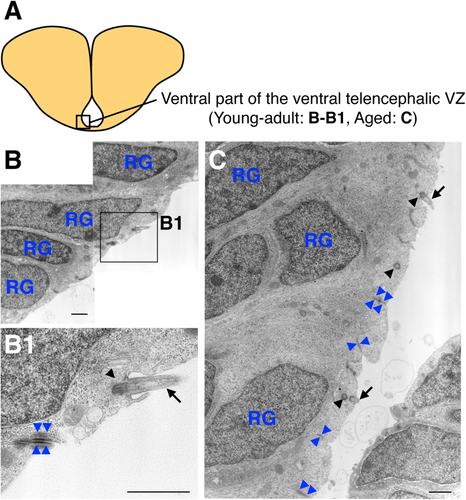
To investigate age-related changes in the cellular composition of the ventral telencephalic VZ, we performed transmission electron microscopy. We previously reported that radial glial cells cover the entire surface of the ventral part of the ventral telencephalic VZ in young-adult fish (Kishimoto et al., 2011; Fig. 4A,B). Here we found that, in aged fish, similar monociliated radial glial cells still covered the ventricular surface of this region (Fig. 4A,C). These radial glial cells had dark cytosol, an elongated nucleus with dense chromatin and nucleolus, and a primary cilium that extended from the basal body (Kishimoto et al., 2011; Fig. 4B1,C). These cells attached to each other by forming adherens junctions at their apical domains (Fig. 4B1,C). These results suggested that the cell composition in the ventral part of the ventral telencephalic VZ is not changed by aging.
In contrast, a change in the cell composition with aging was observed in the dorsal part of the ventral telencephalic VZ (Fig. 5A). For young-adult fish, we observed radial glial cells, neuronal progenitor cells, and migrating new neurons in this region (Fig. 5B). For aged fish, we identified ependymal cells in addition to radial glial cells, neuronal progenitor cells, and migrating new neurons (Fig. 5C–J).
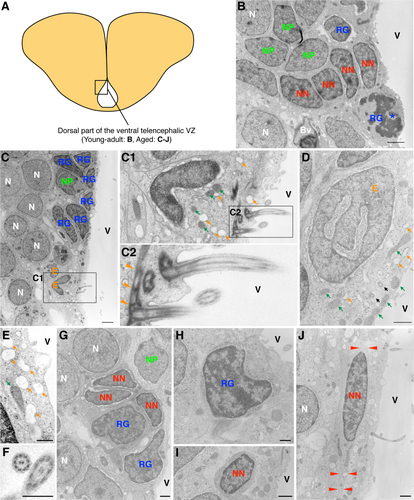
The ependymal cells had a cubic cell body containing many vacuoles, short and dilated rough endoplasmic reticulum cisternae and mitochondria (Fig. 5C–C2,D,E). In their apical domain, these cells presented heavy junctional complexes with adjacent cells. Their surface, exposed to the ventricular cavity, showed multiple cilia with a 9 + 2 microtubule axoneme structure and microvilli (Fig. 5C1,F). The basal bodies associated with these cilia presented complex pericentriolar electron-dense fibers and frequently had mitochondria and small dictiosomes in their vicinity (Fig. 5C2). The nuclei of these cells had dispersed chromatin and were deeply and irregularly invaginated (Fig. 5D). We also occasionally observed ependymal cells with radial cytoplasmic expansion, suggesting that they were in the process of transitioning from a monociliated radial glial cell.
Radial glial cells, although less frequent at this stage, appeared interspersed between ependymal cells contacting the ventricle. They were identified as uniciliated cells, which presented an invaginated nucleus with dark chromatin clumps. Their cytosol contained abundant ribosomes but few organelles (Fig. 5H).
We also identified chain-forming migrating new neurons (Fig. 5G,I,J). These cells showed an elongated cell body with distinct dark cytoplasm, containing abundant free ribosomes and very few organelles. On some occasions, migrating new neurons projected processes with a microtubule-based cytoskeleton, oriented in the long axis of the cell. Their nuclei were irregularly shaped and presented clumped chromatin. In contrast, when cut transversally, these cells presented a smaller area and a regular contour (Fig. 5I).
Neuronal progenitor cells presented a less elongated cell body than the cells identified as migrating new neurons. Their cytosol also was more electron lucent in this cell type, containing mostly polyribosomes. The nuclei of neuronal progenitor cells were, in general, noninvaginated, and they contained more lax chromatin than migrating new neurons but more condensed than that in ependymal cells (Fig. 5C,G).
Along with these cell types, large neurons, a few pyknotic cells, and mitotic cells, were also identified in this region. Taken together, these results suggested that multiciliated ependymal cells emerged in the dorsal part of the ventral telencephalic VZ with aging, forming a cell composition similar to that seen in the mammalian postnatal V-SVZ.
DISCUSSION
Multiciliated cells emerge in the dorsal part of the ventral telencephalic VZ of aged zebrafish
This study shows that multiciliated cells emerge in the ventral telencephalic VZ in aged zebrafish. These multiple cilia had a 9 + 2 microtubule axoneme, a structure typical of motile cilia, and showed coordinated beating. A previous study suggested that fluid flow regulates the ciliary orientation of multiciliated ependymal cells (Guirao et al., 2010). The telencephalic ventricle is a tube-like structure surrounded by the curved ventricular walls of both hemispheres; therefore, CSF flow within the telencephalic ventricle could direct the newly emerged multiple cilia in the rostral direction (Fig. 2C2). Because zebrafish show a long period of brain growth as a result of constitutive neurogenesis (Adolf et al., 2006; Edelmann et al., 2013), it is possible that a brain growth-induced change in the ventricle shape increases the CSF flow within the telencephalic ventricle. The coordinated beating of multiple cilia generates fluid flow toward the rostral direction (Fig. 3), which is parallel to the neuronal migration toward the OB (Adolf et al., 2006; Kishimoto et al., 2011). In both zebrafish and mice, new neurons can migrate toward the OB even before the emergence of multiciliated ependymal cells (Pencea and Luskin, 2003; Adolf et al., 2006; Grandel et al., 2006; Kishimoto et al., 2011). Although the ciliary beating of ependymal cells regulates CSF flow and the directional migration of new neurons in adult mice (Sawamoto et al., 2006), it remains unknown whether it has similar roles in the aged zebrafish. Taken together, these results suggest that the emerged multiciliated cells in aged zebrafish generate CSF flow in the ventral telencephalic ventricle.
Cell composition of the neurogenic niche of aged zebrafish
Our immunohistological and electron microscopy data suggested that the cell composition of the neurogenic niche changes in a region-specific manner. Although the dorsal part of the ventral telencephalic VZ showed the emergence of multiciliated cells, the ventral part retained the monociliated radial glial cells in aged fish (Figs. 4, 5). Mouse Foxj1 is expressed in the precursors of multiciliated ependymal cells (radial glial cells) and activates the transcription of several genes required for ciliogenesis (Jacquet et al., 2009). A recent report showed that foxja1, a homolog of the mammalian Foxj1, is also strongly expressed in the dorsal part of the ventral telencephalic VZ in young-adult zebrafish (Diotel et al., 2015), suggesting that the radial glial transformation into ependymal cells in this region is initiated at the young-adult stage. We found that the emerged multiciliated cells contain dilated cisternae of endoplasmic reticulum and many vacuoles. These cells also showed typical characteristics of multiciliated ependymal cells in mammals such as a cubic cell body and abundant mitochondria in their apical domain (Doetsch et al., 1997; Mirzadeh et al., 2008; Capilla-Gonzalez et al., 2014, 2015). These results suggest that the ependymal cells of aged fish and mice share several features.
Radial glial cells transform into ependymal cells and astrocytes in the neonatal period in the mammalian brain (Merkle et al., 2004; Spassky et al., 2005). We found multiciliated cells with radial glial cell properties in the dorsal part of the ventral telencephalic VZ of aged fish (Fig. 5C1), suggesting that monociliated radial glial cells were transformed into multiciliated ependymal cells. This observation is consistent with a previous report of cells with properties of both radial glial cells and ependymal cells (Lindsey et al., 2012). Alternatively, it is possible that mature ependymal cells have radial cytoplasmic expansion in zebrafish, as reported for other vertebrates (Garcia-Verdugo et al., 2002).
Lindsey et al. (2012) characterized and described several cell types in the telencephalic VZ in the adult zebrafish (Lindsey et al., 2012). According to the nomenclature in that report, the migrating new neurons and neuronal progenitor cells identified in this study (Figs. 4, 5) correspond to the type IVa cells, which have dark cytoplasm and clumped chromatin, and type III cells, which have light cytoplasm and evenly dispersed chromatin, respectively (Lindsey et al., 2012). Taken together, these findings suggest that the cell composition in the dorsal part of the ventral telencephalic VZ of aged fish resembles that in the adult mouse V-SVZ (Fig. 6).
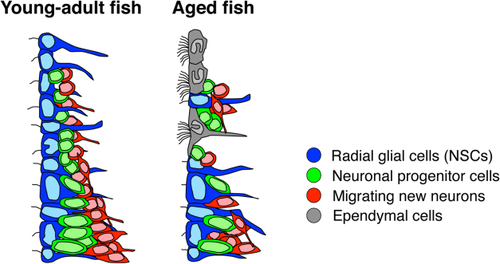
Change in the cellular composition of the neurogenic niche among species
In the mammalian brain, radial glial cells cover the ventricular wall and act as NSCs during the embryonic stage, but they are transformed into ependymal cells and astrocytic NSCs in the V-SVZ immediately after birth (Merkle et al., 2004; Spassky et al., 2005). For zebrafish, our data suggest that monociliated radial glial cells are transformed into ependymal cells at the aged stage in a region-specific manner, leading to a cellular composition similar to that seen in adult mice (Doetsch et al., 1997). Although the mechanism underlying the emergence of ependymal cells remains unknown, these findings raise the possibility that the transformation capacity of radial glial cells is conserved but that its timing is different between fish and mice. The delayed transformation of radial glial cells could account for the higher neurogenic potential of the mature fish brain compared with that of mice. It is possible that the developmental process itself is delayed in zebrafish compared with that in mammals. Alternatively, the maintenance of radial glial cells could be a feature acquired by zebrafish through evolutionary adaptation. Further studies on adult neurogenesis in various species at different ages will clarify the similarities and differences in the cell composition of the neurogenic niche and its regulatory mechanisms, leading to a better understanding of NSC potential in the adult brain.
| Target | Immunogen | Manufacturer, catalog No., species, type | RRID |
|---|---|---|---|
| AceTub | Acetylated tubulin from the outer arm of Strongylocentrotus purpuratus | Sigma, 6-11B-1, mouse monoclonal IgG2b | AB_477585 |
| S100 | S100 isolated from cow brain | DAKO, Z0311, rabbit polyclonal IgG | AB_10013383 |
ACKNOWLEDGMENTS
We are grateful to Dr. Norihito Kishimoto for his contribution to the initial phase of this study, Mr. Taichi Omata for fish care, and members of the Sawamoto laboratory for discussions.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ROLE OF AUTHORS
TO, MS, and KS designed the experiments. KS supervised the study. TO, MS, and CN performed the immunohistochemical analysis. TO, MS, HT, CN, VH-P, AC-S, NK, and JMG-V performed the EM analysis. TO and MS performed the live imaging of ciliary beating and fluid flow. TO, MS, and KS wrote the manuscript.