Fiber connections of the central nucleus of semicircular torus in cyprinids
Abstract
Fiber connections of the presumed auditory portion of the semicircular torus or the central nucleus (TSc) as well as other central and peripheral auditory structures were studied by tract-tracing methods in carp and goldfish. Major ascending projections to the TSc originated from the descending octaval nucleus (DO) and medullary secondary octaval population (SO). Toropetal neurons of the DO were within the saccular terminal zone. A small number of toropetal DO neurons might receive inputs from other inner ear and lateral line endorgans as well. Fibers from the DO terminated in a deep zone of the TSc, while those from the SO in both deep and superficial zones. Afferents to the TSc also arose from the rhombencephalic reticular formation, anterior octaval nucleus, isthmic reticular nucleus, perilemniscular nucleus, medial pretoral nucleus, anterior tuberal nucleus, and central posterior thalamic nucleus. The TSc projected ascending fibers to the medial pretoral nucleus, anterior tuberal nucleus, central posterior thalamic nucleus, and the preglomerular complex (anterior preglomerular nucleus, the caudomedial region of lateral preglomerular nucleus, and a medial zone of medial preglomerular nucleus). These parts of preglomerular complex appear structurally continuous sharing common hodological and cytoarchitectonic features, and thus might be regarded as a single neuronal population. Abundant descending pathways were also noted in the present study. Of particular note is the medial pretoral nucleus, which received fibers from diencephalic auditory nuclei. The nucleus gave rise to indirect descending pathways to a medial zone of the cerebellar crest where dendrites of crest cells in the SO ramify. J. Comp. Neurol. 491:186–211, 2005. © 2005 Wiley-Liss, Inc.
The semicircular torus (TS) is present in nonmammalian vertebrates and is known as the homolog of the inferior colliculus of mammals. The TS of teleosts receives ascending auditory projections in addition to lateral line inputs (for review, see Meek and Nieuwenhuys, 1998). The sacculus is considered the major acoustic receptor in teleosts, although all otolithic endorgans can potentially contribute to hearing (Popper, 1983; McCormick, 1999). The lateral line organ of most teleosts is mechanosensory, but in some teleost lineages an additional electrosensory division of the lateral line system is present, which can detect electric signals (for review, see Meek and Nieuwenhuys, 1998). Inputs from these receptor organs reach the rhombencephalon and are then conveyed to the TS. Thus, the TS is regarded as a relay station of at least two or three modalities depending on teleostean lineages. The presumed auditory toral nucleus is called the central nucleus of semicircular torus (TSc) in most teleosts.
Cyprinids belong to the otophysan taxon and thus possess a chain of Weberian ossicles and associated ligaments that couple the swim bladder with the sacculus (for review, see Popper, 1983). Cyprinids, together with other species with some forms of specialized peripheral auditory system, are called “hearing specialists.” Species without such specializations are referred to as “hearing generalists.” Cyprinids are hence good experimental subjects, and some studies have been conducted on the central auditory connections. A study in carp (Echteler, 1984) and especially another report in goldfish (McCormick and Hernandez, 1996) have revealed a general pattern of ascending auditory pathways to the TS in cyprinids. The TSc receives major ascending projections from the descending octaval nucleus (DO) and the secondary octaval population (SO). In addition, the anterior octaval nucleus projects to the TSc, at least in carp. Physiological studies have been also performed in cyprinids, supporting that the TSc is a mesencephalic auditory structure (carp: Echteler, 1984, 1985a, b; goldfish: Lu and Fay, 1993; Ma and Fay, 2002).
Auditory pathways from the rhombencephalon through the torus to the diencephalon, however, have not been studied thoroughly in any one cyprinid species. In goldfish, neurons afferent to the TSc are present in dorsomedial (DOdm) and intermediate (DOi) zones of the DO (McCormick and Hernandez, 1996) and probably within the saccular terminal zone (McCormick and Braford, 1994). Data that morphologically support the auditory nature of the DO zones afferent to the TSc are lacking in carp. The presumed auditory zone of the DO is known to project to the SO only in goldfish (McCormick and Hernandez, 1996). Diencephalic connections of the TSc have been reported only in carp: reciprocal connections with the central posterior thalamic nucleus and anterior tuberal nucleus, and an efferent projection to the ventromedial thalamic nucleus (Echteler, 1984).
In goldfish and catfish the connections of SO or a comparable neuronal population (a part of the medial auditory nucleus in catfish) have been reported based on SO injection experiments that involved other structures as well (McCormick and Hernandez, 1996; Finger and Tong, 1984). The precise connections of the SO may be studied by more refined specific injection experiments. In particular, it remains to be determined whether the DO and SO project in the same pattern to the TSc. Also, the SO is covered dorsally by the cerebellar crest, where Purkinje cell-like neurons or crest cells of the SO extend their dorsal dendrites to receive inputs from parallel fibers (McCormick and Hernandez, 1996; McCormick, 1999). The granular eminence and preeminential nucleus are known to send fibers to the cerebellar crest of electrosensory lateral line lobe of electrosensory teleosts (for review, see Meek and Nieuwenhuys, 1998) and to the cerebellar crest zone overlying the medial nucleus of rhombencephalic octavolateral area (catfish: Finger and Tong, 1984; goldfish: McCormick and Hernandez, 1996). However, The origin(s) of inputs to the rostromedial zone of the cerebellar crest (i.e., to dorsal dendrites of the crest cells of SO) remains unknown so far as we know.
A presumed higher-order auditory center is the preglomerular complex (PG), which is a major source to the telencephalon and can be divided into several parts or nuclei (Meek and Nieuwenhuys, 1998). It is crucial to clarify the precise fiber connections of this complex to understand the organization of ascending sensory systems. Although Echteler (1984) and Murakami et al. (1986b) reported toral projections to the PG in carp, the origin of nuclei within the TS and terminal distribution pattern within the PG have not been precisely determined. In another otophysan catfish the ventrolateral nucleus of TS (TSvl) is known to project to the lateral preglomerular nucleus (PGl), but the TSc may not send fibers to the PG (Striedter, 1991). Auditory inputs may reach the PGl via the medial pretoral nucleus in otophysans (Striedter, 1991, 1992). In contrast, the auditory torus has been reported to send fibers to the PG in a mormyrid (Pollimyrus: Kozloski and Crawford, 1998). It remains to be studied in cyprinids whether the TSc projects directly to the PG unlike catfish.
As enumerated above, connections of the TSc and other auditory structures require further studies in cyprinids. We analyzed connections of a variety of central structures and of saccular, lagenar, and posterior lateral line nerves in carp and goldfish, with a special reference to the TSc and PG. We describe both ascending and descending auditory pathways.
Abbreviations
-
- AO
-
anterior octaval nucleus
-
- APd
-
dorsal part of the area pretectalis
-
- APv
-
ventral part of the area pretectalis
-
- BDA
-
biotinylated dextran amine
-
- CC
-
cerebellar corpus
-
- CM
-
mamillary corpus
-
- CP
-
central posterior thalamic nucleus
-
- CrC
-
cerebellar crest
-
- dV
-
descending tract of trigeminal nerve
-
- DO
-
descending octaval nucleus
-
- DOdm
-
dorsomedial zone of DO
-
- DOi
-
intermediate zone of DO
-
- DOm
-
DOdm + medial part of DOi
-
- DOv
-
ventral zone of DO
-
- EG
-
granular eminence
-
- flm
-
medial longitudinal fascicle
-
- FLM
-
nucleus of flm
-
- fr
-
retroflexal fascicle
-
- GP
-
granule population (McCormick and Hernandez, 1996)
-
- hc
-
horizontal commissure
-
- HB
-
habenula
-
- IP
-
interpeduncular nucleus
-
- IRN
-
isthmic reticular nucleus
-
- LC
-
locus coeruleus
-
- LCa
-
caudal lobe of cerebellum
-
- lfb
-
lateral forebrain bundle
-
- LI
-
inferior lobe
-
- ll
-
lateral lemniscus
-
- mca
-
anterior mesencephalocerebellar tract
-
- MO
-
magnocellular octaval nucleus
-
- MPN
-
medial pretoral nucleus
-
- NAT
-
anterior tuberal nucleus
-
- NCLI
-
central nucleus of the inferior lobe
-
- NDLI
-
diffuse nucleus of the inferior lobe
-
- NI
-
isthmic nucleus
-
- NLT
-
lateral tuberal nucleus
-
- NLV
-
lateral valvular nucleus
-
- NLVa
-
anterior part of NLV
-
- NLVc
-
central part of NLV
-
- NLVpl
-
posterolateral part of NLV
-
- NLVpm
-
posteromedial part of NLV
-
- NM
-
medial nucleus of rhombencephalic octavolateral area
-
- NPE
-
preeminential nucleus
-
- NPL
-
perilemniscular nucleus (Wullimann and Northcutt, 1988)
-
- NPT
-
posterior thalamic nucleus
-
- NR
-
nucleus ruber (Goldstein, 1905)
-
- NRL
-
nucleus of lateral recess
-
- NIII
-
oculomotor nucleus
-
- NVd
-
descending trigeminal nucleus
-
- NVm
-
trigeminal motor nucleus
-
- NVs
-
trigeminal sensory nucleus
-
- NVIIm
-
facial motor nucleus
-
- od
-
dorsal optic tract
-
- ov
-
ventral optic tract
-
- pc
-
posterior commissure
-
- PG
-
preglomerular complex
-
- PGa
-
anterior preglomerular nucleus
-
- PGl
-
lateral preglomerular nucleus
-
- PGlc
-
caudomedial region of PGl
-
- PGlr
-
rostrolateral region of PGl
-
- PGm
-
medial preglomerular nucleus
-
- pLL
-
posterior lateral line nerve
-
- Pm
-
magnocellular preoptic nucleus
-
- Pp
-
parvocellular preoptic nucleus
-
- PSm
-
magnocellular part of superficial pretectal nucleus
-
- PSp
-
parvocellular part of superficial pretectal nucleus
-
- pTGN
-
preglomerular tertiary gustatory nucleus
-
- SCN
-
suprachiasmatic nucleus
-
- sg
-
secondary gustatory tract
-
- SG
-
subglomerular nucleus
-
- SGN
-
secondary gustatory nucleus
-
- SO
-
secondary octaval population
-
- SOv
-
ventral division of SO
-
- SV
-
vascular sac
-
- T
-
tangential octaval nucleus
-
- td
-
torodiencephalic tract
-
- tg
-
tertiary gustatory tract
-
- TE
-
telencephalon
-
- TL
-
longitudinal torus
-
- TLa
-
lateral torus
-
- TO
-
optic tectum
-
- TS
-
semicircular torus
-
- TSc
-
central nucleus of TS
-
- TSvl
-
ventrolateral nucleus of TS
-
- VC
-
valvula of cerebellum
-
- VCl
-
lateral lobe of VC
-
- VCm
-
medial lobe of VC
-
- VM
-
ventromedial thalamic nucleus
-
- III
-
oculomotor nerve
-
- VII
-
facial nerve
-
- VIIm
-
facial motor root
-
- VIIs
-
facial sensory root
-
- VIII
-
octaval nerve
MATERIALS AND METHODS
A total of 22 carp Cyprinus carpio and 25 goldfish Carassius auratus (Cyprinidae, Teleostei) of both sexes were used for the present study. The carp and goldfish ranged from 11–30 and 5–16 cm in standard length, respectively. The fish were obtained from commercial sources and maintained in aquaria at 26–29°C and fed trout or goldfish food once every few days. Twenty carp and 21 goldfish were used for in vivo tract-tracing study, two goldfish for in vitro tract-tracing study, and two carp and two goldfish for a carbocyanine dye (1,1′, dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate: DiI) study of fixed brains. The present study was performed under the official Japanese regulations for research on animals and those of our institution.
Tract-tracing studies of nonfixed brains
In vivo tract-tracing study.
Animals were anesthetized by immersing in water containing 150 mg/l 3-aminobenzoic acid ethyl ester (MS222; Sigma, St. Louis, MO) and were positioned in a device for the physical restraint. During surgery the gills were perfused with 75 mg/l MS222 solution to maintain the anesthetic condition of the fish and for artificial respiration. A dorsal portion of the cranium was removed by a dental drill and the adipose tissue filling the cranial cavity was aspirated to expose the brain. Biocytin (Sigma) was injected as dry crystals with an insect pin. Biotinylated dextran amine (BDA: molecular weight 3,000; Molecular Probes, Eugene, OR) was iontophoretically injected. A glass microelectrode was filled with BDA diluted to ∼1% with phosphate buffered saline (PBS, pH 7.4), and direct cathodal current (5 μA) was passed through the electrode for 10–30 minutes (2 seconds on/2 seconds off). The numbers of samples are the follows: 1) TSc: five carp and eight goldfish; 2) SO/medial cerebellar crest: five carp and six goldfish; 3) medial portions of DO: seven carp and two goldfish; 4) saccular nerve and lagenar nerve: one carp; 5) posterior lateral line nerve: two carp and one goldfish; and 6) medial pretoral nucleus: four goldfish. Posterior lateral line nerve experiments were performed because primary afferents of the lateral line nerve have been reported to terminate in the goldfish DO (Puzdrowski, 1989). For injections to the TSc and medial pretoral nucleus the tectal commissure was incised and the cerebellar valvula was shifted medially to expose the ventricular surface of the torus. After tracer injections the opening of cranium was closed by means of a cap formed by dental cement affixed with acrylic glue.
Animals were maintained postoperatively in a tank for 2–4 days, then deeply anesthetized with MS222 (300 mg/l) and perfused through the conus arteriosus with saline followed by 4% paraformaldehyde in 0.1 M PB, pH 7.4. The brains were removed from the skull and postfixed in a fresh solution of the same fixative overnight.
In vitro tract-tracing study.
Tract-tracing experiments in vitro were performed after procedures reported elsewhere (Yamamoto et al., 1998; Sawai et al., 2000). Fish were deeply anesthetized by immersing in 300 mg/l MS222 solution. After decapitation the brains were quickly removed from the skull and placed in a standard Krebs-Ringer solution. Small crystals of biocytin were applied with an insect pin to the TSc in one goldfish and SO in one goldfish. After tracer applications the brains were transferred into containers filled with 30 ml fresh Krebs-Ringer solution and the air was replaced by 95% O2: 5% CO2 before sealing the container with a cap. The containers were kept at room temperature and gently shaken during incubation. The solution and the oxygen mix were changed every 30 minutes. After 6–7 hours of incubation the brains were fixed by immersing in 4% paraformaldehyde in 0.1 M PB (pH 7.4) overnight.
Tissue processing.
Fixed brains were cryoprotected by immersing in 0.1 M PB containing 20% sucrose for 6 hours or longer, embedded in 5% agar (Sigma, Type IV) containing 20% sucrose, and cut frontally at 40–50 μm on a cryostat. Thaw-mounted sections on glass slides were washed with 0.05 M PBS containing 0.3% Triton X-100 and incubated with ABC solution (1:100; Sigma, ABC elite kit) overnight at room temperature. After three 10-minute washes in 0.1 M PBS the sections were reacted with 0.5% diaminobenzidine (DAB) solution containing 0.04% nickel ammonium sulfate and 0.01% H2O2, counterstained with 0.025% cresyl violet, dehydrated, and coverslipped.
Carbocyanine dye (DiI) experiments of fixed brains
The DiI was used for tract-tracing experiments on the saccular and lagenar nerves because of technical difficulties of in vivo and in vitro injections. One carp and one goldfish were used for DiI application experiments to the saccular nerve, and one carp and one goldfish for lagenar nerve experiments. The fish were perfused as in in vivo specimens. The brains were removed from the skull and postfixed in a fresh solution of the same fixative for 2 days. Small crystals of DiI were inserted into the targeted nerve and the insertion site was covered by 5% gelatin (Sigma type A) to avoid translocation of the crystals. The brains were maintained in the dark in the fixative for 2 weeks at 37°C. The brains were then embedded in 8% gelatin (Sigma, type A), fixed for 3 days, and cut into 80-μm frontal sections on a microslicer (D.S.K., DTK-3000). Sections were mounted on slides with 50% sucrose in 0.1 M PB (pH 7.4), coverslipped, and observed with an AX80 microscope (Olympus, Lake Success, NY) equipped with epifluorescence illumination system.
Cytoarchitecture
In addition to tract-tracing materials, Nissl- and Bodian-stained materials available in our laboratory (Ito, 1978) were used to analyze the cytoarchitecture of the cyprinid brain.
Nomenclature
For the octavolateral areas in the rhombencephalon and mesencephalon we adopted the nomenclature on goldfish of McCormick and Braford (1994) and McCormick and Hernandez (1996). We used the terminology of Ito and Yoshimoto (1990) on the carp brain for the subdivisions of lateral valvular nucleus. For diencephalic structures we basically used the nomenclature of Braford and Northcutt (1983) on the goldfish brain. Other terms are also introduced where necessary.
RESULTS
Cytoarchitecture of the preglomerular complex
The PG has been subdivided into several parts in the goldfish (Peter and Gill, 1975; Braford and Northcutt, 1983). The subdivisions, unfortunately, do not agree with cytoarchitectonic as well as hodological data as studied in the present study. Therefore, we first describe the organization of PG, based on the analysis of Nissl- and Bodian-stained materials in conjunction with tract-tracing results, for clarity of the following descriptions.
The organization and cytoarchitecture of the PG are similar in carp (Fig. 1) and goldfish. The most rostral preglomerular structure is the anterior preglomerular nucleus (PGa) of Braford and Northcutt (1983), which occupies a position ventrolateral to the optic tract and torodiencephalic tract. The PGa is composed of small and round neurons (about 5–6 × 5–8 μm in carp) stained darkly in Nissl-stained materials (Fig. 1E). Slightly caudal to the rostral pole of the PGa appears the PGl. For some rostrocaudal distance the PGa and PGl are seen in the same frontal planes, with the PGl located lateral to the PGa (Fig. 1A). Caudally, preglomerular tertiary gustatory nucleus (previously termed nucleus glomerulosus) and medial preglomerular nucleus (PGm) appears ventral to the PGl (Fig. 1B). Our cytoarchitectonic analysis clearly indicates that the PGl can be further divided into rostrolateral (PGlr; Fig. 1A–C) and caudomedial (PGlc; Fig. 1C,D) regions. The PGlc is composed of small and round neurons (about 5–6 × 5–8 μm: carp, Fig. 1F), which are darkly stained, similar to PGa neurons. Neurons in the PGlr possess totally different morphological features. They are medium-sized and usually more elongate (about 7–12 × 8–15 μm: carp), and are lightly stained (Fig. 1F,G). Caudally, the PGa shifts ventrally and becomes smaller in frontal sections. At these levels emerging PGlc and PGm are dorsally and ventrally adjacent to the PGa, respectively. The PGm is composed of small neurons like those in the PGa and PGlc (Fig. 1H). The boundaries between the three parts composed of small neurons are quite difficult to determine. Further caudally, the commissural preglomerular nucleus appears caudomedial to the PGlc.
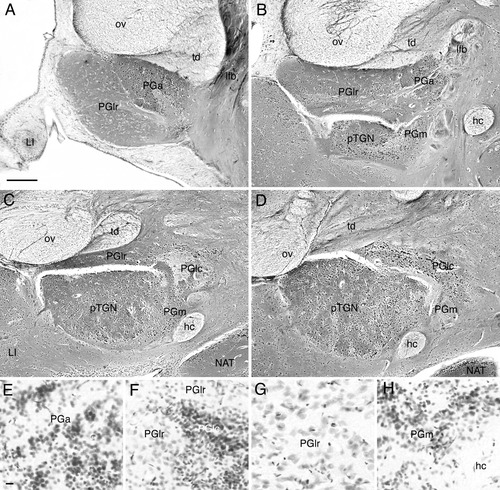
The organization and cellular morphology of different parts of preglomerular complex as shown in Bodian-stained (A–D) and Nissl-stained sections (E–H) of carp. A–D are arranged in the rostrocaudal order. Medial is to the right in all panels. Abbreviations as in list . Scale bars = 200 μm in A (applies to A–D); 10 μm in E (applies to E–H).
Tract-tracing studies revealed that the PGa receives abundant fibers from the TSc (Fig. 2A,B). The terminal zone of TSc fibers continued caudally from the PGa to the PGlc and a medial zone of PGm (Fig. 2C,D), although the terminal zone became small in frontal sections at junction levels between the auditory preglomerular parts (Fig. 2B). More caudally, the terminal zone became larger again in the PGlc and the medial zone of PGm (Fig. 2C,D), where the former replaces the rostrolateral region or PGlr.
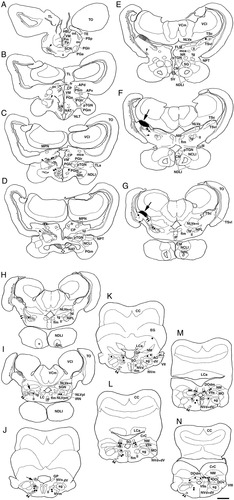
A–N: Chartings of labeled structures following injection of BDA into the central nucleus of the semicircular torus in carp. Filled circles, thin lines, thick lines (B–D) and small dots indicate labeled cells, axons, dendrites, and terminals, respectively. Circles with straight line (L–N) indicate labeled crest cells and their dorsal dendrites of the secondary octaval population and descending octaval nucleus. Black areas pointed by large arrow and checkered areas in E–I indicate injection site and diffusion area, respectively. Levels A–N are arranged rostrocaudally. Arrowhead: labeled fibers in torodiencephalic tract; double arrowhead: labeled fibers in torobulbar tract; small arrow: a branch from torobulbar tract to the granule population and secondary octaval population. Abbreviations as in list . Scale bar = 1 mm in N (applies to A–N).
Tract-tracing studies
Biocytin and BDA were well transported both anterogradely and retrogradely. The data obtained by the two tracers were identical qualitatively, although the superiority of biocytin to BDA in anterograde transport and the superiority of BDA than biocytin in retrograde transport were noticed. Studies in in vivo and in vitro brains yielded almost identical results. Therefore, we present together the results obtained by the two tracers either in vivo or in vitro. Results obtained in the carp and goldfish were virtually the same, indicating similar organization of auditory pathways through the brain stem to the diencephalon in these closely related fishes. The following descriptions thus apply to both species unless otherwise noted.
Injections to the central nucleus (TSc).
In three carp and three goldfish cases injections were confined within the TSc (Figs. 2F,G, 3A). Tracers injected to the TSc did not diffuse into subjacent TSvl, and labeled structures were seen almost throughout the TSc (Figs. 2E–I, 3B). In the TSvl only sparsely labeled cells and terminals were present.
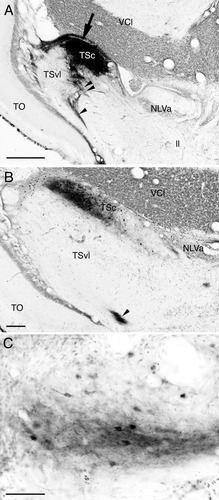
Injection site (A), boundary between the central nucleus and ventrolateral nucleus (B), and labeled terminals and cells in the medial pretoral nucleus (C) after BDA injection to the carp central nucleus. Medial is to right in all panels. Note in B a sharp boundary between the central nucleus and ventrolateral nucleus, and absence of labeled structures in the ventrolateral nucleus. Large arrow: injection site; arrowhead: torodiencephalic tract; double arrowhead: torobulbar tract. Abbreviations as in list . Scale bars = 500 μm in A; 200 μm in B; 50 μm in C.
Labeled fibers in the TSc continued rostromedially to the medial pretoral nucleus of Finger and Tong (1984). Labeled terminals as well as cells were seen in the nucleus (Figs. 2C,D, 3C). Some labeled fibers crossed the midline through the posterior commissure to terminate in the contralateral medial pretoral nucleus (Fig. 2B–D). Occasionally labeled cells and terminals were seen in the contralateral TSc (Fig. 2E,F). In the midbrain tegmentum a few neurons and terminals were labeled lateral to the lateral lemniscus (Fig. 2F). Only sparsely labeled terminals but no cells were seen in the ipsilateral optic tectum, mainly in the stratum album centrale (Fig. 2A–I). Tectal injections do not label cells in the TSc (Yamamoto and Ito, unpubl. obs.).
Many labeled fibers entered the lateral lemniscus and ran caudally (Fig. 2E). At isthmic levels labeled ovoid or multipolar neurons were seen in or medially adjacent to the lateral lemniscus with ipsilateral dominance (Figs. 2I, 4A). These cells appear to correspond to neurons of isthmic reticular nucleus (Bell, 1981; Kozloski and Crawford, 1998; also see McCormick, 1999). At similar levels labeled fibers were seen to cross the midline to reach labeled cells in the contralateral nucleus. Some of the labeled fibers in the lateral lemniscus crossed the midline at levels of the rhombencephalon (Fig. 2L). Labeled neurons were seen in the anterior octaval nucleus, mainly contralateral to the injection site (Figs. 2K, 4B). In the goldfish, however, labeled neurons were seen in the nucleus only after large injections that affected both the TSc and TSvl.
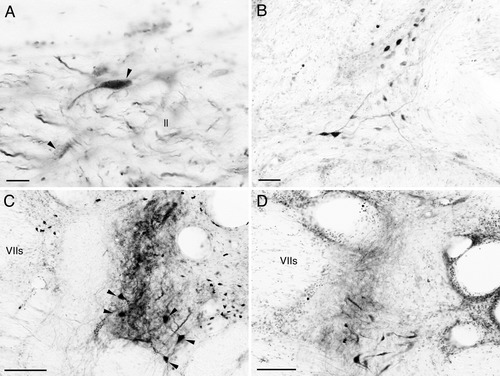
A–F: Labeled neurons and terminals in the isthmus (A) and rhombencephalon (B–F) after BDA injections in the central nucleus (TSc) in carp. Medial is to the left in B and right in other panels. A: Labeled neurons of the isthmic reticular nucleus (arrowheads) in the ipsilateral lateral lemniscus (ll). Labeled fibers in the lemniscus are also seen. B: Labeled cells in the anterior octaval nucleus contralateral to the injection site. C: Labeled cells and terminals in the ipsilateral secondary octaval population. Labeled neurons of intermediate division are indicated by arrowheads. Injection site was in rostral TSc. Note the presence of labeled structures in a medial zone of the nucleus. D: Labeled structures in the secondary octaval population at similar rostrocaudal level as E. Injection site was in middle to caudal TSc. Labeled structures are in a lateral zone of the nucleus. Other abbreviations as in list . Scale bars = 20 μm in A; 50 μm in B; 100 μm in C,D.
Most of the labeled fibers in the lateral lemniscus could be traced to the SO and DO, which were the main sources to the TSc (Fig. 2K–N). Slightly larger numbers of labeled neurons were present in the ipsilateral SO than the contralateral nucleus. Three divisions of SO as reported by McCormick and Hernandez (1996) in goldfish were labeled also in carp: 1) dorsal division with Purkinje cell-like large neurons or crest cells that possess a dorsal dendrite extending to a medial zone of cerebellar crest, and a ventral dendrite that reached the intermediate division; 2) ventral division with large fusiform or occasional multipolar neurons near or rostral to the crossing fibers of lateral lemniscus; and 3) intermediate division with spherical and medium-sized neurons (Fig. 4C). Most of the fusiform neurons in the ventral division were oriented dorsoventrally, extending dorsal dendrites into the intermediate division and ventral dendrites toward the labeled torobulbar fibers running dorsally to terminate in the SO. Occasionally horizontally oriented neurons were also present. Rostrally, similar fusiform neurons were seen to the level of a group of granular neurons capping the rostral pole of the SO or the granule population of McCormick and Hernandez (1996). Rostrally located fusiform neurons were regarded as a part of the ventral division of SO. A possible topographical organization of the SO-TSc pathway was also noted. When the injection sites were in the rostral TSc, labeled cells were mainly present in a medial zone of the SO (Fig. 4C). In contrast, injections to middle to caudal TSc resulted in labeled cells in a lateral zone of the SO (Fig. 4D).
Most of the labeled cells in the DO were medium-sized and ovoid (Fig. 5). Occasionally large fusiform neurons of crest cell type were also seen (Fig. 5A). Many labeled DO neurons were seen dorsally and laterally adjacent to the facial sensory root (Fig. 5), i.e., restricted in the DOdm and DOi of McCormick and Braford (1994). At middle levels of the nucleus, labeled fusiform cells in the DOdm were also seen near the midline ventral to the cerebellar crest in the carp. The axis of these neurons was horizontal and extended dendrites that cross the midline to the contralateral nucleus in addition to dendrites in the ipsilateral side (Fig. 5E). The vast majority of somata and dendrites of the labeled DOi neurons were restricted in the medial part of this zone. Labeled neurons in the DOdm also tended to aggregate in the ventromedial portion of the zone or close to the facial sensory root. The DOdm and medial part of DOi, where labeled neurons and dendrites were seen, will be referred to as medial DO (DOm) for convenience when we refer to these zones cumulatively. A few labeled cells were seen in a more lateral part of the DOi (Fig. 5B,F). No labeled neurons were encountered in the lateralmost region of DOi or in the ventral zone of the DO of McCormick and Braford (1994). Labeled neurons in the DOm were abundant in the contralateral nucleus, which is different from the laterality of the SO neurons. The DOm-TSc pathway might also be organized topographically. When the injection sites were in the rostral TSc, labeled cells mainly appeared caudal and ventral portions of the DOm (Fig. 5A–D). In contrast, injections to middle to caudal TSc resulted in labeled cells mainly in rostral and dorsal portions (Fig. 5F).
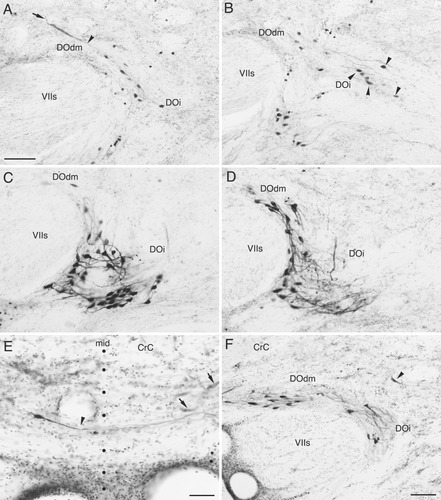
Labeled cells in the descending octaval nucleus (DO) after BDA injections to the carp central nucleus. In A–D and F, contralateral DO is shown and medial is to the left. In E midline (mid) is indicated by dots. A–D: Serial sections but with one section omitted between B and C. Injection site was in rostral torus. In A, a crest cell is seen with a dorsal dendrite (arrow) and a ventral dendrite (arrowhead). Note that labeled neurons are only seen in the dorsomedial (DOdm) and intermediate zones (DOi). Labeled cells and their dendrites in the DOdm and DOi occupy ventromedial and medial region of the zone (i.e., close to the facial sensory root), respectively. Labeled cells are numerous in the caudoventral zone of DOi (C,D) of this specimen. There are much fewer cells in sections rostral to A. Also note that a few labeled cells are present more laterally somewhat apart from the major population (arrowheads in B). E: A labeled neuron in the ipsilateral DOdm extends a medial dendrite (arrowhead) that cross the midline into the contralateral DOdm. Dendrites are also seen in the ipsilateral side. Arrows indicate weakly labeled cells in the contralateral DOdm. F: A section from a different specimen from the one shown in A–D. Injection site was in middle to caudal torus. Section level corresponds roughly to that of A. Note the presence of more labeled cells than in A in portions of DOdm dorsally adjacent to VIIs. In this carp case, there were moderate numbers of labeled cells (including crest cells) in the DO rostral to F, while only a few labeled cells were noticed in more caudoventral portions of the DO (i.e., portions with many labeled cells in C,D). Arrowhead indicates a laterally located cell apart from the major medial population. Abbreviations as in list . Scale bars = 100 μm in A (applies to A–D),F; 50 μm in E.
Another population of labeled fibers originated from the injected TSc, coursed ventromedially, and descended to the rhombencephalon (torobulbar tract: Figs. 2F–N, 3A, double arrowhead). Labeled fibers in the torobulbar tract gave rise to a terminal zone in the isthmus bordered medially by the lateral lemniscus and dorsally by the isthmic nucleus (Fig. 2G,H). This area corresponds to the perilemniscular nucleus of Wullimann and Northcutt (1988), a cerebellopetal nucleus identified in sunfish and goldfish. Dense terminals were seen only in a dorsal portion of this nucleus (Fig. 6A), where labeled neurons were also present (Fig. 2G,H). Some of the fibers running close to the perilemniscular nucleus coursed dorsally to terminate in the lateral valvular nucleus (Fig. 2H,I). Terminals were restricted to a lateral portion of the nucleus, a zone that appears a caudal extension of the anterior part of lateral valvular nucleus of Ito and Yoshimoto (1990). Terminals in the lateral valvular nucleus showed a peculiar cup-shaped morphology (Fig. 6B), which characterizes terminals in this nucleus (Yoshimoto and Ito, 1993).
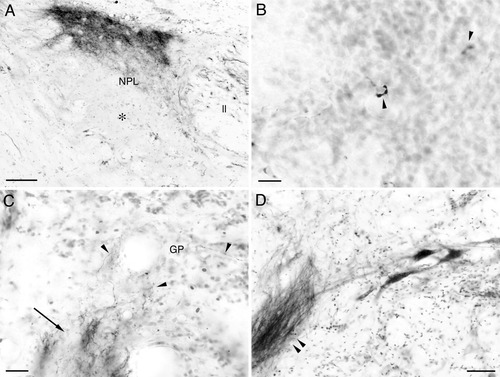
Labeled terminals and cells associated with the torobulbar tract after BDA injections to the central nucleus of semicircular torus (TSc) in carp. Medial is to the right in all panels. A: The perilemniscular nucleus (NPL) ipsilateral to the injected TSc. Terminals are dense in the dorsal part of the nucleus, while the ventral part is free of terminals (asterisk). B: Labeled terminals (arrowheads) in the lateral valvular nucleus ipsilateral to the injection. Level of section approximately corresponds to that of Figure 2K. Terminals show peculiar cup-shaped morphology and appear to encircle a target neuron. C: Labeled fibers in the bundle descending to the secondary octaval population (arrow). Some fibers depart the bundle to terminate in the granule population ipsilateral to the injection (arrowheads). D: Labeled fibers in the torobulbar tract (double arrowhead) and labeled reticular neurons ipsilateral to the injection. Scale bars = 100 μm in A; 50 μm in B,D; 20 μm in C.
At the level of the secondary gustatory nucleus a compact bundle of labeled fibers departed the torobulbar tract proper and turned dorsomedially (Fig. 2I: small arrow). This bundle then traveled caudally through an area ventrolateral to the granule population of McCormick and Hernandez (1996), providing terminals to the population of granular cells (Figs. 2J,K, 6C). The labeled fibers continued further caudally and entered the SO from its rostral aspect to terminate in the nucleus (Fig. 2L).
Other labeled fibers in the torobulbar tract proper continued further caudally and gave off terminals in the reticular formation (Fig. 2J,K). Labeled neurons were seen in the rhombencephalic reticular formation dorsomedial to the torobulbar tract and ventromedial to the lateral lemniscus (Figs. 2J,M,N, 6D). They were larger than the fusiform neurons in the ventral division of SO. Each neuron had a ventrolateral dendrite extending toward the labeled fibers of torobulbar tract (Fig. 6D) and a dorsomedial dendrite (destination was unclear). Similarly located neurons were also encountered contralaterally.
At the level of the SO most fibers in the torobulbar tract turned dorsally to terminate densely in the SO (Fig. 2L,M). Terminals were seen in the intermediate and ventral divisions of SO and crest cells of the dorsal division can also receive fibers on their ventral dendrites. The terminal zone continued sparsely into a restricted ventral region of the DOm (i.e., ventral to most neurons afferent to the torus; Fig. 2N). It was not clear whether the terminal zone is a caudal extension of the dendritic field from the ventral division of SO. Terminals in the SO showed the same topography as labeled cells in the SO (Fig. 4C,D). Terminals were also seen in the contralateral SO. However, terminating fibers in the contralateral SO could be traced back to retrogradely labeled neurons of the contralateral DOm, indicating that DOm axons projecting to the TSc send collaterals to the SO. Similar collateral axon terminals from the DOm may be also present in the ipsilateral SO, but this could not be confirmed definitively due to numerous labeled fibers. A few fibers in the torobulbar tract continued further caudally to terminate in the ipsilateral reticular formation (Fig. 2M,N).
A third population of labeled fibers originated from the injected TSc (Fig. 2F,G, arrowhead), the majority of which projected to the diencephalon (torodiencephalic tract). The labeled fibers coursed rostrally through the ventrolateral margin of the semicircular torus (Figs. 2E, 3A,B, arrowhead), some of which turned medially and gave terminals in the PGlc (Figs. 2C,D, 7A), while not in the PGlr. Labeled fibers also traveled ventrally to terminate in a medial zone of the PGm (Fig. 7A,D,E) and in the anterior tuberal nucleus. More rostrally, the terminal zone in the PGlc and PGm continued to the PGa (Fig. 7E). Labeled terminals in the preglomerular nuclei are quite large and showed strange morphologies (Fig. 7B,C, described in more detail below). Other medially oriented fibers reached a terminal zone in the central posterior thalamic nucleus of Braford and Northcutt (1983) or a caudal portion of the dorsomedial thalamic nucleus (Figs. 2B,C, 8A). Large labeled neurons were also present in a lateral zone of the nucleus (Figs. 2C, 8B). These neurons were multipolar and the dendrites could be classified into three populations: lateral, medial, and rostral. The lateral dendrites approached labeled fibers and terminals of the torodiencephalic tract (Fig. 8B). The medial dendrites reached the caudomedial zone of the central posterior thalamic nucleus, where smaller unlabeled cells and labeled terminals were present. The rostral dendrites were extremely long, coursed rostroventrally, and finally entered the anterior tuberal nucleus, where numerous labeled terminals were seen (Figs. 2B,C, 8C).
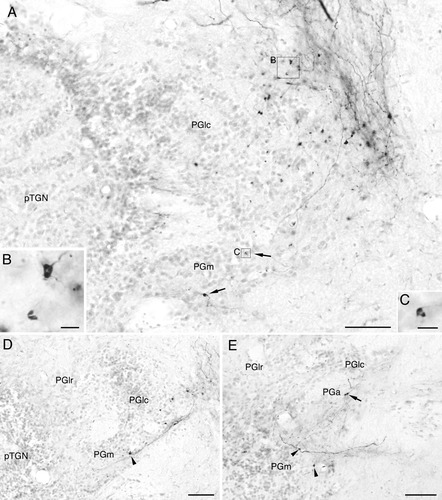
Labeled terminals in the preglomerular complex after biocytin injection to the central nucleus of the semicircular torus (TSc) in goldfish. All panels show structures ipsilateral to the injection; medial is to the right. A: Labeled terminals in the caudomedial region of lateral preglomerular nucleus (PGlc) and in a medial zone of medial preglomerular nucleus (PGm: arrows). B: Higher magnification of boxed area “B” in A showing terminals in the PGlc. Terminals appear like a donut or a pair of semicircular structures sandwiching a narrow unlabeled slit. C: Higher magnification of boxed area “C” in A, showing a terminal in the medial zone of PGm. D: Labeled terminals in the PGlc and in the medial zone of PGm (arrowhead) in a section rostral to A. The rostrolateral region of lateral preglomerular nucleus (PGlr) lies lateral to the PGlc at this section level. E: Labeled terminals in the PGm (arrowhead) and caudalmost PGa (arrow) in a section rostral to D. The rostral pole of the PGlc is seen dorsally adjacent to the PGa, and the PGm is ventrally adjacent to the PGa. The three preglomerular parts thus appear continuous. Other abbreviations as in list . Scale bars = 50 μm in A,D,E; 5 μm in B,C.
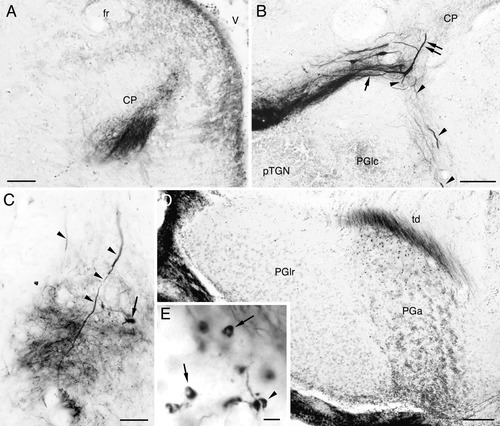
Labeled structures in the diencephalon after biocytin injection to the central nucleus of the semicircular torus (TSc) in goldfish (A,B) and BDA injection to the TSc in carp (C–E). All panels show structures ipsilateral to the injection, and medial is to the right. A: Labeled terminals in the central posterior thalamic nucleus (CP). B: Labeled large cells in the lateral region of CP. Lateral (arrow), medial (double arrow), and rostral (arrowheads) dendrites are visible. Labeled fibers from torodiencephalic tract form a dense terminal zone surrounding the lateral dendrites and continue medially to the smaller cell zone of CP and to the caudomedial region of lateral preglomerular nucleus (PGlc). C: Labeled terminals in the anterior tuberal nucleus. A labeled cell is also seen (arrow). Rostral dendrites (arrowheads) of labeled large neurons in the CP are seen to enter the nucleus dorsally. D: Low-magnification view of labeled terminals in the anterior preglomerular nucleus (PGa). E: Labeled terminals in the PGa. They appear like a donut (arrows) or a pair of semicircular structures sandwiching a narrow unlabeled slit (arrowhead). Other abbreviations as in list . Scale bars = 50 μm in A,C; 100 μm in B,D; 5 μm in E.
Other labeled fibers in the torodiencephalic tract continued further rostrally through an area dorsal to the PGlr (Fig. 2B), then shifted ventromedially, and provided abundant labeled fibers with large swellings in the PGa (Figs. 2A,B, 8D,E). The swellings were quite large but were regarded as terminals for the following reasons: 1) they were smaller (about 3 μm: carp) than the somata of PGa neurons (5–6 × 5–8 μm: carp); and 2) their peculiar morphology was unlike that of somata (Fig. 8E). Very frequently, each swelling appeared like a donut with an unlabeled hole (about 1 μm or less in diameter: carp) or a pair of semicircular structures sandwiching a narrow unlabeled slit (about 1 μm or less in width and 2–3 μm in length: carp). Thus, the labeled structures are most likely presynaptic terminals surrounding dendrites of the target PGa neurons and thus exhibit different morphologies, depending on the cutting angles. Terminals in the PGlc and a medial zone of PGm also showed similar morphologies (Fig. 7B,C). Some of the labeled fibers continued medial to the PGa (Fig. 2A), and then turned caudally to terminate densely in the anterior tuberal nucleus (Figs. 2B–D, 8C). Some fibers crossed the midline (Fig. 2A) to terminate in the contralateral anterior tuberal nucleus (Fig. 2B–D) and TSc (Fig. 2E). Small numbers of labeled neurons were seen bilaterally in the anterior tuberal nucleus (Figs. 2B,C, 8C).
A few large labeled neurons (presumed eurydendroid cells) were present in the contralateral cerebellum close to the granular eminence (Fig. 2K). In one goldfish case, labeled terminals were seen in the supracommissural and ventral parts of the ventral telencephalic area (not charted).
Injections to the DOm.
In three carp and one goldfish cases tracers were injected into the DOm with minimal diffusion to neighboring structures (Figs. 9A, 10D). In these cases, labeled terminals, spherical cells, and crest cells were present in the contralateral DOm (Fig. 10D), indicating reciprocal connections between the nuclei on both sides. Labeled fibers from the DOm ascended through the bilateral lateral lemniscus (Fig. 10B,C). Some of the labeled fibers turned dorsally and terminated in the SO with ipsilateral dominance (Figs. 9B, 10C). Terminals were seen in intermediate and ventral divisions, but crest cells can receive this projection on their ventral dendrites. A few lightly labeled cells were also present in the intermediate and ventral divisions of SO (Figs. 9B, 10C). At the same level a few labeled cells were seen in the bilateral descending trigeminal nucleus and magnocellular octaval nucleus (Fig. 10C). Labeled neurons in the magnocellular octaval nucleus were smaller than gigantic cells that give rise to descending spinal projections (Yamamoto and Ito, unpubl. obs.). Along the lateral lemniscus occasional labeled terminals were seen on both sides and a few labeled fusiform cells were also present in the terminal field at the levels of granule population of McCormick and Hernandez (1996) (Fig. 10B). They were regarded as fusiform neurons of the ventral division of SO (see Discussion). At mesencephalic levels labeled fibers finally shifted laterally and terminated exclusively in the TSc with contralateral dominance (Figs. 9C, 10A). Terminal distribution in the TSc was not uniform. Terminals were present only in the deep or ventral zone of this nucleus, forming a terminal layer (Figs. 9C, 10A).
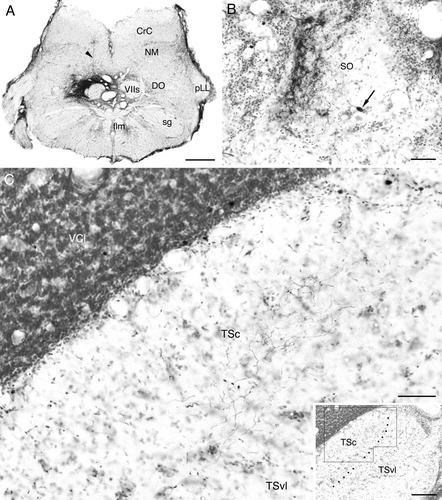
Labeled structures after BDA injection to the descending octaval nucleus (DO) in carp. A: Injection site. Injected tracer is limited almost perfectly within the dorsomedial zone and medial region of intermediate zone of DO. The needle track (arrowhead) is dorsal to the injection site, which is visible because of accumulation of glial cells, not by backward diffusion of the tracer along the track. B: Labeled terminals and a cell (arrow) in the secondary octaval population ipsilateral to the injection. Medial is to the right. C: Labeled terminals in the central nucleus of the semicircular torus (TSc) contralateral to the injection. Terminals are restricted to the ventral (or deeper) zone of the nucleus. Inset indicates the boundary between the TSc and ventrolateral nucleus (TSvl) at a lower magnification, and the area shown in C. Medial is to the left. Other abbreviations as in list . Scale bars = 500 μm in A; 50 μm in B,C; 200 μm in inset.
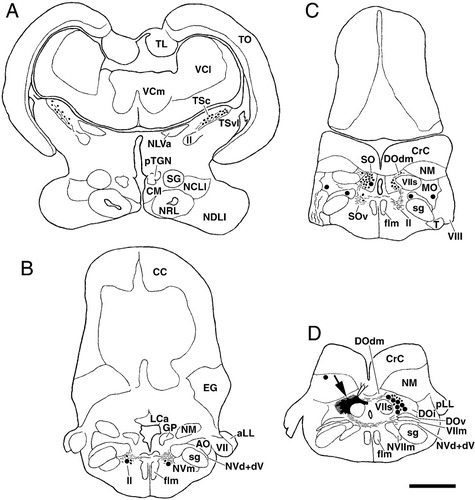
A–D: Chartings of labeled structures following injection of BDA into the medial portion of descending octaval nucleus in carp. Filled circles, thin lines, and small dots indicate labeled cells, axons, and terminals, respectively. Black areas and checkered areas pointed by large arrow in D indicate injection site and diffusion area, respectively. Dorsal dendrites of crest cells of descending octaval nucleus are also depicted as lines from the injection site in D. Levels A–D are arranged rostrocaudally. Abbreviations as in list . Scale bar = 1 mm in D (applies to A–D).
Injections to SO and medial cerebellar crest.
In two carp cases the injection was restricted within the SO (excluding the ventral division) and did not involve the overlying cerebellar crest (Figs. 11A, 12F). In these cases labeled spherical and ventral fusiform cells (but not crest cells) as well as terminals were seen in the contralateral SO, indicating reciprocal connections between the bilateral nuclei (Fig. 12F). Crest cells may also receive commissural projections on their ventral dendrites. Many labeled ovoid cells as well as a few crest cells were observed in the DOm with slight ipsilateral dominance (Fig. 12G). The distribution and morphology of labeled DOm neurons were similar to those labeled by TSc injections (Fig. 11B, cf. Fig. 5). Terminals were also present in the bilateral DOm (Fig. 12G). A few small cells were labeled bilaterally in the medial nucleus of the rhombencephalic octavolateral area (Fig. 12F,G). Labeled ascending fibers coursed through the bilateral lateral lemniscus (Fig. 12C–E). Occasionally labeled terminals as well as fusiform cells were seen in or near the lateral lemniscus at the level of granule population (Fig. 12E), similar to DOm injection cases. Sometimes the ventral dendrites of the labeled cells could be followed to reach a reticular formation zone where torobulbar fibers and terminals were labeled after TSc injections. They were regarded as the rostral continuation of fusiform neurons of ventral division of SO (see Discussion). A small fraction of labeled fibers coursed rostrally along the ventrolateral margin of the medulla oblongata and terminated sparsely in a dorsal zone of the perilemniscular nucleus (Fig. 12C). Labeled terminals were seen medial to the lateral lemniscus on both sides or in the isthmic reticular nucleus (Fig. 12C). A few labeled fibers were seen in the preeminential nucleus. Labeled fibers along the lateral lemniscus reached the torus and terminated in the TSc with a slight ipsilateral dominance (Figs. 11C, 12B,C). No terminals were encountered in the TSvl. In contrast to DOm injection cases, labeled fibers terminated in both dorsal and ventral zones of the TSc (Fig. 11C). Also different from the DOm injection cases, labeled fibers continued rostral to the TS and terminated in the medial pretoral nucleus (Figs. 11D, 12A). Some of the fibers crossed the midline through the posterior commissure, and fewer terminals were seen in the contralateral nucleus (Fig. 12A). Labeled cells were observed in the ipsilateral medial pretoral nucleus (Figs. 11D, 12A). There were no labeled cells in the TSc.
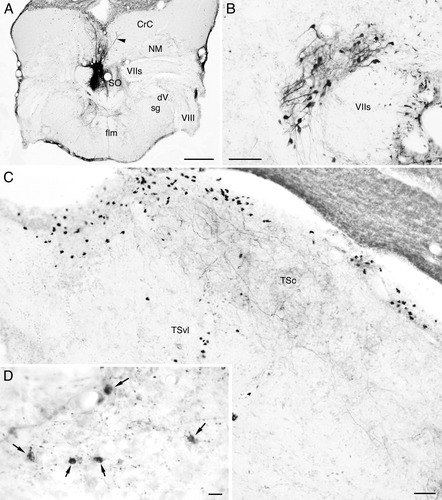
Labeled structures after BDA injection to the secondary octaval population (SO) in carp. Structures in B–D are ipsilateral to the injection, and medial is to the right. A: Injection site. Injected tracer is restricted within the SO (dorsal and intermediate zones). Labeled structures in the cerebellar crest (CrC) are dorsal dendrites of crest cells of the SO, which took up the tracer from their somata and ventral dendrites. Note invasion of a dorsal dendrite to the contralateral CrC (arrowhead). B: Labeled cells in the medial portion of the descending octaval nucleus. C: Labeled terminals in the central nucleus of the semicircular torus (TSc). Terminals are seen in dorsal (or superficial) and ventral (or deep) zones of the nucleus. Black ovoid structures accumulated mainly in the periventricular zone are stained by nonspecific reaction, not labeled cells. D: Labeled cells (arrows) and terminals (visible as small dots) in the medial pretoral nucleus. Other abbreviations as in list . Scale bars = 500 μm in A; 100 μm in B; 50 μm in C; 10 μm in D.
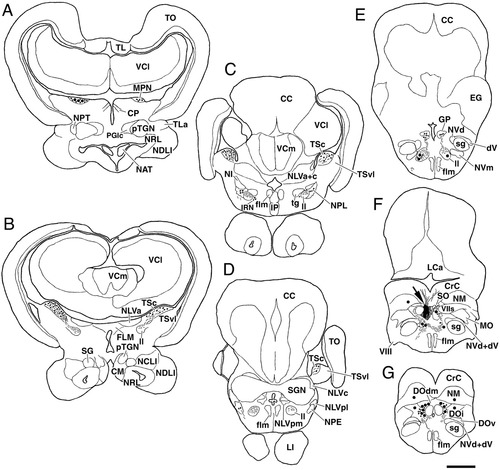
A–G: Chartings of labeled structures following injection of BDA into the secondary octaval population (SO) in carp. Filled circles, thin lines, and small dots indicate labeled cells, axons, and terminals, respectively. Black areas and checkered areas pointed by large arrow in F indicate injection site and diffusion area, respectively. Dorsal dendrites of crest cells of SO are also depicted as lines in the cerebellar crest (CrC) in F. Levels A–G are arranged rostrocaudally. Other abbreviations as in list . Scale bar = 1 mm in G (applies to A–G).
In one goldfish case the injection site was confined within a medial zone of the cerebellar crest overlying the SO (Fig. 13). This injection labeled densely many crest cells of SO, while only a few crest cells of the DO were faintly labeled. In this case labeled cells were seen in the granule population of McCormick and Hernandez (1996) (Fig. 13). A few labeled cells continued from the granule population to the rostrolateral part of the granular layer of the caudal lobe. Another group of labeled cells was seen in a lateral zone of the granular eminence (Fig. 13). Labeled granular cells were scattered between the granule population and granular eminence. Also, a few labeled cells continued rostroventrally from the granular eminence to the preeminential nucleus. Labeled neurons in the above-mentioned structures were dominant in the side ipsilateral to the injection. In contrast to cases with injection limited to the SO proper, very few labeled terminals were seen in the contralateral SO. A labeled crest cell was seen in the contralateral SO, which most likely became labeled directly from its dorsal dendrite invading the cerebellar crest of the injected side. Few terminals were present in the DOm. Labeled axons of the crest cells exited the SO laterally and then traveled ventrally along the lateral margin of the facial sensory root. Most axons ascended through the lateral lemniscus to terminate in the TSc with slight ipsilateral dominance. Terminals were seen in both dorsal and ventral zones of the TSc, similar to injection cases to the SO proper. Labeled terminals were also seen in the medial pretoral nucleus with ipsilateral dominance. No labeled neurons were seen in the nucleus, which is distinct from SO injection cases.
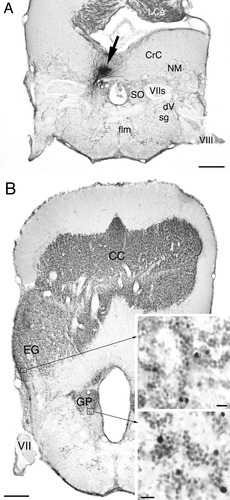
Labeled structures after BDA injection to the cerebellar crest (CrC) in goldfish. A: Injection site (arrow). Injected tracer is restricted within the medial zone of CrC overlying the secondary octaval population (SO). B: Low-magnification photomicrograph showing the locations of the granule population (GP) and granular eminence (EG) ipsilateral to the injection. Upper inset shows labeled cells in a ventrolateral part of the granular eminence and lower inset those in the granule population at higher magnifications. Other abbreviations as in list . Scale bars = 500 μm in A,B; 10 μm in insets.
In other carp and goldfish cases, injected tracers involved both the cerebellar crest and SO proper ventral to the crest. The results of these cases were in accordance with the specific injection cases to the SO proper and medial cerebellar crest described above.
Injections to the saccular, lagenar, and posterior lateral line nerves.
The in vivo case including both saccular and lagenar nerves in a carp resulted in only a few labeled fibers and was not analyzed further. Selective DiI insertions to the saccular and lagenar nerve in the carp labeled the targeted nerve without affecting other branches of the octaval nerve. Labeled terminals were seen only in the ipsilateral brain. The most dense terminal zone of the saccular nerve was an area dorsolateral to the facial sensory root (Fig. 14A,B). Labeled fibers also ascended further dorsomedially and terminated in more dorsal and rostral zones of DOdm. Transneuronally labeled cells including crest cells were occasionally seen in and close to the terminal field. The saccular terminal zone thus coincided almost perfectly with the somatic and dendritic zones of DOm neurons afferent to the TSc (cf. Fig. 5). The lagenar nerve fibers terminated laterally adjacent to the saccular terminal zone (Fig. 14C,D). These two terminal zones may partially overlap each other. A few terminals were also seen in the anterior octaval nucleus subsequent to injections of either nerve. Basically the same pattern of connections was seen in goldfish, confirming a previous study (McCormick and Braford, 1994). Some labeled fibers proceeded further rostrally to terminate in the granular eminence and in or ventrolaterally adjacent to the granule population.
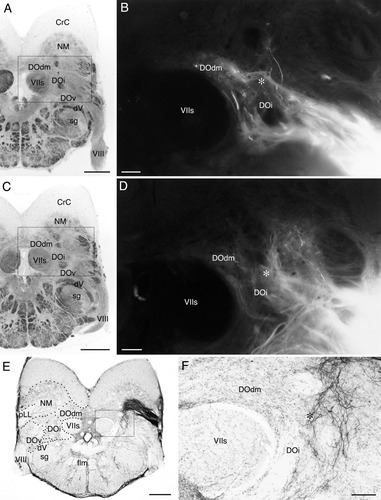
Labeled terminals in the descending octaval nucleus (DO) after DiI application to the saccular (A,B) and lagenar (C,D) nerves, and after biocytin injection to the posterior lateral line nerve (E,F) in carp. The levels of sections correspond roughly to that of Figure 5B. A–D: Brightfield photomicrographs of A and C indicate the areas enlarged in B and D that show DiI labeled terminals, respectively. Note that the saccular terminal zone is dorsolaterally adjacent to the facial sensory root (VIIs) and coincides very well with the distribution of toropetal neurons of the DO (cf. Fig. 5). The lagenar terminal zone is lateral to the saccular terminal zone (overlap may be present). E,F: Boxed area in E is enlarged in F. Asterisks: a zone of possible overlapping inputs from the three nerves. Abbreviations as in list . Scale bars = 500 μm in A,C,E; 100 μm in B,D,F.
After tracer injections to the posterior lateral line nerve, most of the labeled fibers terminated in the medial nucleus of the rhombencephalic octavolateral area. Some of the labeled fibers coursed ventromedially toward the DO and terminated in a lateral zone of the DOi, and terminals may partially overlap with saccular and lagenar terminal zones (Fig. 14, asterisk). Most labeled terminals appeared lateral to the somatic and dendritic zones of most DO neurons afferent to the TSc (Fig. 14E,F, cf. Fig. 5). However, a fraction of DO neurons may fall within the overlapping zone of saccular, lagenar, and lateral line terminals (Fig. 5B,F, arrowheads). Labeled lateral line terminals were not seen in the DOdm, unlike that reported in a previous study of goldfish (Puzdrowski, 1989).
Injections to the medial pretoral nucleus.
In addition to the medial pretoral nucleus, ventrally adjacent structures were slightly affected by these injections. We thus focus the following descriptions on labeled structures in auditory-related nuclei, which represent presumed connections of the medial pretoral nucleus.
Labeled terminals and cells were seen in the superficial zone of the TSc (ipsilateral dominance: Fig. 15A), confirming reciprocally the results of the TSc injection study.
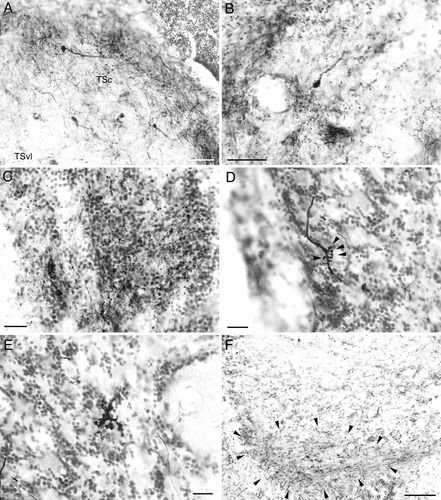
Labeled structures after tracer injections to the medial pretoral nucleus in goldfish. Ipsilateral side is shown and medial is to the right in all panels. A: Labeled cells and terminals in the central nucleus of the semicircular torus (TSc). Labeled terminals are dense in the most superficial layer of the TSc. B: Labeled terminals and a cell in the secondary octaval population (intermediate division). C: Labeled terminals in the granule population. D: A labeled fiber in the ventrolateral zone of the granular eminence. Cudgel-shaped terminals protrude from the fiber (arrowheads). E: A large terminal structure in the ventrolateral zone of the granular eminence showing complex morphology with irregular swellings. F: Labeled terminals in the rostrolateral region of lateral preglomerular nucleus. Note the restricted distribution of terminals in the ventral zone (encircled by arrowheads). Scale bars = 50 μm in A,B; 20 μm in C–E; 100 μm in F.
Three to four compact bundles of labeled fibers emerged from the injection site, which were close to but distinct from the lateral lemniscus. All but one bundle descended through the rhombencephalon, assuming a position ventral to the lateral lemniscus and finally turned dorsally to terminate in the bilateral SO (ipsilateral dominance: Fig. 15B). All three types of SO neurons were also labeled in the bilateral nuclei. These observations agree with the results of SO injection experiments. Some fibers contributed sparse terminals in the ventralmost region of DOm. One of the compact labeled bundles took a more dorsal course toward the granule population of McCormick and Hernandez (1996), where numerous labeled terminals of mossy fiber type were seen (Fig. 15C). Labeled fibers in the granule population continued dorsolaterally to supply terminals in a lateral zone of the granular eminence, a zone where labeled cells were seen after injections to the medial cerebellar crest. Terminals in the eminence were large, with quite bizarre morphologies (Fig. 15D,E). Fewer terminals were seen in the granule population and granular eminence contralateral to the injection. Labeled cells as well as terminals were seen in the dorsal region of the perilemniscular nucleus with ipsilateral dominance.
Labeled terminals were seen in the ipsilateral PGlr restricted in the ventralmost zone (Fig. 15F). Labeled cells and terminals were seen in the anterior tuberal nucleus and central posterior thalamic nucleus. Labeled terminals were also seen in the PGa, a medial zone of PGm, and PGlc ipsilateral to the injected side. These terminals showed similar morphologies to those labeled by auditory toral injections. The possibility thus cannot be excluded that some TSc neurons send fibers to the medial pretoral nucleus and collaterals to these preglomerular portions.
DISCUSSION
The present study indicates that presumed auditory information is relayed to the TSc mainly by the SO and DO, which largely agrees with previous studies in cyprinids (carp: Echteler, 1984; goldfish: McCormick and Braford, 1994; McCormick and Hernandez, 1996) as well as in other teleosts (catfishes: Finger and Tong, 1984; McCormick, 2001; mormyrid: Kozloski and Crawford, 1998; batrachoidids: Bass et al., 2000, 2001; cichlid: O'Marra and McCormick, 1999; also see McCormick, 1999). In addition, the present study suggests organized laminar terminal patterns of secondary and tertiary auditory fibers in the TSc, which has not been reported in previous studies. Ascending as well as descending interconnections between auditory nuclei were revealed in greater detail with a number of new findings on the hodology, cellular morphology, and possible topographic relationships between auditory structures. Furthermore, diencephalic connections of the TSc were clarified in the present study in cyprinids. The TSc projects not only to the dorsal thalamus and anterior tuberal nucleus but also to the PG. Fibers from the TSc have a broad distribution within the PG, terminating in the PGa, a medial zone of PGm, and PGlc. This finding would help a better understanding of the PG afferent to the telencephalon. Ascending and descending pathways are schematically illustrated in Figure 16 based on the present study. In the following sections we discuss in more detail auditory structures in the caudal to rostral order followed by a section on descending pathways.
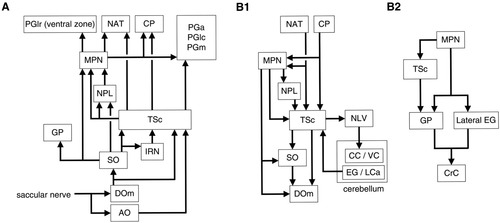
Schematic diagrams of auditory pathways. A: Ascending auditory pathways to the diencephalon. The descending octaval nucleus (DO) may receive additional (although minor) inputs from the lagenar and lateral line nerves. The anterior octaval nucleus (AO) also receives inputs from other otolithic organs (McCormick and Braford, 1994; present study). B1: Descending pathways and cerebellar auditory pathways. B2: Descending pathways to the zone of cerebellar crest (CrC) overlying the secondary octaval population (SO). Other abbreviations as in list . Connections of the medial pretoral nucleus (MPN) have to be confirmed further in a strict sense.
Toropetal zone of the descending octaval nucleus or DOm
The sacculus is regarded as a specialized and major auditory receptor organ in cyprinids, although other otolithic organs might also contribute to hearing (Popper, 1983; McCormick, 1999). In the carp DOm, the distribution of the somata and dendrites retrogradely labeled by TSc injections coincided almost perfectly with the saccular terminal zone (see Figs. 5, 14). These results are in accordance with previous studies in goldfish (McCormick and Braford, 1994; McCormick and Hernandez, 1996). The DOm of the present study corresponds to the DOdm and DOi of these authors. Bilateral DOm are reciprocally connected in carp, as has been reported in goldfish (McCormick and Hernandez, 1996) and in a batrachoidid (Edds-Walton, 1998).
The present study suggests that the lagenar and lateral line nerves terminate mainly lateral to the saccular terminal zone, in accordance with previous studies in goldfish (McCormick and Braford, 1994; Puzdrowski, 1989). Terminal zones of the three nerves may overlap, where a small number of toropetal neurons were found lateral to the major neuronal population (present study). Thus, the cyprinid DOm may also relay minor auditory inputs from the lagena and lateral line inputs to the torus. The laterally located neurons of the cyprinid DOm may correspond to those of dorsolateral division of DO in a batrachoidid (Bass et al., 2000), which also receive octaval and lateral line inputs and project to the TSc. The present findings of labeled cells in the descending trigeminal nucleus and magnocellular octaval nucleus after tracer injections to the DOm apparently suggest convergent somatosensory and vestibular inputs to the DOm. An alternative explanation, however, is the slight tracer spread to more lateral portions of DO, which is a probable vestibular and lateral line zone (McCormick and Braford, 1994; present study) and is more likely to have such afferent connections.
The DOdm or its homolog is well developed in “hearing specialists” (Finger and Tong, 1984; McCormick and Braford, 1993; 1994; McCormick, 1997) but is present also in “hearing generalists” (McCormick, 1992; O'Marra and McCormick, 1999; for review, see McCormick, 1999). Spherical and crest cells are present in the DOdm in both goldfish and carp (McCormick and Braford, 1994; McCormick and Hernandez, 1996; present study). However, the present study suggests a small difference in the nuclear organization between carp and goldfish. In carp, DOdm neurons near the midline extend their dendrites to the contralateral nucleus in addition to those in the ipsilateral nucleus. A convergence of bilateral saccular inputs in the DOdm is also known in mormyrids (Kozloski and Crawford, 1998; McCormick, 1999).
Secondary octaval population (SO)
Based on tract-tracing experiments a secondary octaval nucleus was identified in a pioneering study on auditory pathways and was termed the medial auditory nucleus in catfish (Finger and Tong, 1984). However, subsequent studies suggest that the nucleus is in fact composed of primary and secondary nuclei that correspond to DOdm and SO of cyprinids, respectively (McCormick and Braford, 1993; McCormick and Hernandez, 1996). In goldfish the SO is composed of three divisions, dorsal (crest cells), intermediate (spherical), and ventral (fusiform), as revealed by a detailed cytoarchitectonic as well as a hodological study by McCormick and Hernandez (1996). Similarly, three divisions are present also in other “hearing specialists” (carp: present study; catfish: McCormick and Hernandez, 1996; mormyrid: Kozloski and Crawford, 1998; clupeid; McCormick, 1997; also see McCormick, 1999). The intermediate division may be lacking in a batrachoidid (midshipman: Bass et al., 2000) and a percomorph (cichlid: O'Marra and McCormick, 1999), i.e., in “hearing generalists.” Thus, the presence of the intermediate division may be linked with the specialization in the peripheral auditory system and with the well-developed DOdm. Studies in other teleost groups as well as in nonteleost fishes, however, are important to understand the evolution of the SO more precisely.
A previous study in goldfish suggests that the SO receives inputs from the bilateral DOm and sends fibers to the TSc with slight ipsilateral dominance (McCormick and Hernandez, 1996). Specific injections to the SO and DOm of the present study confirmed these connections. In addition, the present study indicates that the bilateral SO are reciprocally connected. The catfish medial auditory nucleus is known to project to the medial pretoral nucleus (Finger and Tong, 1984). The present study indicates that only the secondary nucleus or SO projects to the medial pretoral nucleus in cyprinids. The medial auditory-medial pretoral projection in catfish may originate from the neuronal component that corresponds to the SO in cyprinids.
Labeled cells were found in the medial nucleus of the octavolateral area and in a lateral zone of DOi in the present SO injection experiment. These results, however, may be due to tracer uptake from commissural fibers of these nuclei that course dorsal and ventral to the SO (McCormick and Hernandez, 1996; Yamamoto and Ito, unpubl. obs.).
In the present study we injected tracer specifically to a medial zone of cerebellar crest overlying the SO, an experiment that has not been performed in teleosts so far as we know. Such injections clarified efferents of crest cells of the SO. The crest cells gave rise to only ascending projections. In contrast, injections to the SO proper resulted in labeling descending and commissural fibers as well. These results suggest functional differentiation of the three divisions of SO.
Anterior octaval nucleus
In the present study toral injections labeled neurons in the anterior octaval nucleus, where sparse projections of saccular and lagenar nerves were found. Thus, the anterior octaval nucleus may serve as a minor auditory relay nucleus to the torus. This nucleus may also relay inputs from other inner ear endorgans (McCormick and Braford, 1994). Auditory toral injections did not label cells in the anterior octaval nucleus in goldfish (McCormick and Hernandez, 1996) and a marine catfish, Arius (McCormick, 2001), although these toral projections are present in carp (Echteler, 1984; present study) and a different catfish, Ictalurus (Finger and Tong, 1984). In the present study, only large injections including the TSc and TSvl labeled cells in the anterior octaval nucleus in goldfish. In batrachoidids the anterior octaval nucleus appears to send no or few fibers to the auditory torus (Edds-Walton, 1998; Bass et al., 2000, 2001). The anterior octaval nucleus is known to project to the torus in mormyrids (Bell, 1981; Kozloski and Crawford, 1998), Xenomystus (Braford et al., 1993), and a percomorph rockfish (Murakami et al., 1986a), while not in a different species of percomorph (cichlid: O'Marra and McCormick, 1999). Thus, the anterior octaval nucleus-toral pathway is present in most teleosts with varying degrees of development.
Toropetal neurons along the lateral lemniscus
Toropetal neurons have been observed along the lateral lemniscus in various teleosts (e.g., Echteler, 1984; Murakami et al., 1986a; McCormick and Hernandez, 1996; Kozloski and Crawford, 1998; present study; also see McCormick, 1999). These neurons appear to represent multiple populations. The present study adds new hodological data on these neurons and suggests they may be classified into four populations or nuclei as follows.
Most of the toropetal neurons found along the lemniscus in the rhombencephalon may be SO neurons, as judged from the similar morphology, connections, and continuous distribution with the ventral division of SO. The ventral division of the SO has been suggested to continue rostrally to the dorsal and intermediate divisions (McCormick and Hernandez, 1996). In the present study, TSc and SO injections labeled fusiform neurons along the lemniscus rostral to the SO. These neurons possess dorsal and ventral dendrites similar to neurons of the ventral division of SO. Tracer injections to the DOm labeled similar fusiform neurons as well as terminals along the lateral lemniscus, suggesting that the rostrally located fusiform neurons receive auditory inputs and project back to the DOm as do SO neurons. Although we do not have direct evidence that neurons labeled by toral injections and those by SO or DOm injections are the same neuronal population, it is likely that rostrally located fusiform neurons afferent to the TSc belong to the SO.
Large toropetal neurons were seen in the rhombencephalon ventromedial to the lateral lemniscus, i.e., in the medial reticular formation. These labeled cells were usually larger than ventral fusiform neurons of the SO, and their axes were oriented horizontally (see Fig. 6D). These neurons are hence regarded as reticular neurons, although they have dendrites extending to the labeled toromedullary tract, as do ventral fusiform neurons of the SO.
The third toropetal population is found at the isthmic level in or medially adjacent to the lateral lemniscus. These neurons are ovoid, form a more compact population than more caudal fusiform neurons, and are not labeled by SO or DOm injections. The neuronal group probably corresponds with isthmic reticular nucleus in mormyrids (Bell, 1981; Kozloski and Crawford, 1998). Labeled terminals were seen close to these toropetal reticular neurons after SO injections in the present study. The isthmic reticular nucleus in cyprinids is hence probably an auditory nucleus.
A fourth toropetal population is the perilemniscular nucleus, which is laterally adjacent to the lateral lemniscus and is known as cerebellopetal in goldfish and green sunfish (Wullimann and Northcutt, 1988, 1989). Perilemniscular neurons were labeled in the present materials of TSc injections, in which there was no tracer spread to the overlying cerebellar valvula. We thus conclude that this nucleus projects to the TSc, as has been reported in a marine catfish (McCormick, 2001). Interestingly, toropetal cells and torofugal terminals were restricted in the dorsal zone of the nucleus in the present study, suggesting the presence of subnuclear connectional differences.
Lateral valvular nucleus
Labeled terminals were observed in the lateral valvular nucleus after TSc injections in the present study. This connection has not been reported in previous studies in cyprinids. In a mormyrid, auditory toral fibers are known to terminate in the isthmic granular nucleus (Kozloski and Crawford, 1998), which is a probable homolog of the lateral valvular nucleus (Meek and Nieuwenhuys, 1998). Both superficial and deep zones of the torus (i.e., presumed lateral line and auditory zones) project to the lateral valvular nucleus in a percomorph tilapia (Yang et al., 2004). It is also known in a mormyrid that the isthmic granular nucleus (or ganglion isthmi) receives fibers also from an electrosensory toral nucleus (Haugede-Carre, 1979). Thus, auditory as well as lateral line inputs appear to reach the lateral valvular nucleus, which in turn sends fibers to the cerebellum (Meek and Nieuwenhuys, 1998). Interestingly, toral fibers form large terminals surrounding the somata of target neurons in the lateral valvular nucleus (referred to as “cup-shaped” in cyprinids or “end-bulb” in mormyrids), suggesting that these terminals exert strong influences on the activity of target neurons.
Central nucleus of the semicircular torus (TSc)
The present TSc injection materials resulted in sparse labeled cells and terminals in the TSvl, suggesting that the toral nuclei are connectionally quite independent. It remained unclear whether the few labeled cells in the TSvl send axons and/or dendrites to the TSc in the present study. Toral neurons with dendrites extending in both auditory and lateral line nuclei have been reported in the TSvl of a nonelectrosensory “hearing generalist,” a batrachoidid (toadfish: Edds-Walton and Fay, 2003). The organization of the TSc has also been studied in detail in batrachoidids based on toral injection materials (Bass et al., 2000, 2001). There are differences in the degree of development and the organization of the TS between nonelectrosensory cyprinids (McCormick and Hernandez, 1996; present study) and batrachoidids. In batrachoidids the TSc occupies only a thin periventricular layer of the torus and appears less well developed than in cyprinids. Some neurons of the batrachoidid TSc extend dendrites to the TSvl, suggesting convergence of auditory and lateral line inputs on these neurons (Bass et al., 2000; Weeg and Bass, 2001), which was not observed in cyprinids (present study). Also different from the cyprinids, the TSc and TSvl are reciprocally connected in batrachoidids (Bass et al., 2000; Weeg and Bass, 2001). Detailed analyses on the organization of the TS in other teleosts are required to correlate the differences to the degree of specialization in the peripheral auditory system.
The present study suggests laminar organization of the TSc with respect to auditory afferent fibers. The DOm projects only to the deep (or ventral) zone of TSc, while the SO to both superficial and deep zones, which can be a morphological substrate important for auditory processing in the toral nucleus. In goldfish the lateral line nucleus (TSvl) is present ventral to the TSc (McCormick and Hernandez, 1996). Therefore, the cyprinid TS as a whole may be organized in a laminar fashion. Laminar organization of the torus is also known in other nonelectrosensory teleosts (Ito, 1974). In some electrosensory fishes the electrosensory portion adds to the torus as nuclear masses rather than as additional layers (see Meek and Nieuwenhuys, 1998), and the reason for the organizational differences is an interesting question to be explored.
The present toral injection experiments suggest that the rostrocaudal axis of the TSc is reflected to the caudoventral to rostrodorsal axis of DOm and to the mediolateral axis of the SO. It has been reported that the auditory torus of carp is tonotopically organized (Echteler, 1985b). The axis of tonotopy is rostromedial to caudolateral (rostromedial: high frequency, caudolateral: low frequency), an axis that appears to correspond to the rostrocaudal topography of the TSc suggested in the present study. To establish firmly the presence and pattern of topographical relationship and to correlate it to the tonotopy of the torus, however, we need to inject tracers into different portions of TSc more systematically.
Diencephalic ascending connections of the TSc
The present study indicates that the TSc projects to the central posterior thalamic nucleus and to the anterior tuberal nucleus. These results basically agree with previous studies (carp: Echteler, 1984; batrachoidid: Bass et al., 2000, 2001). In a mormyrid, Kozloski and Crawford (1998) found an auditory toral projection to the posteroventral nucleus of Bell (1981). The homolog of the posteroventral nucleus remains uncertain in cyprinids. Toral projections to the ventromedial thalamic nucleus have been reported in carp and rockfish (Echteler, 1984; Murakami et al., 1986a), but were not observed in the present study.
Auditory toral projections to the PG were found in the present study. Such toral projections have not been reported in a different otophysan catfish (Striedter, 1991), although they have been observed in some teleosts (mormyrid: Kozloski and Crawford, 1998; batrachoidids: Bass et al., 2000, 2001). The auditory preglomerular part has been identified as the PGl in batrachoidids. The present study indicates that the PGa, a medial zone of PGm, and the caudomedial region of the PGl or PGlc receive auditory toral fibers in cyprinids. Toral terminals were seen as a continuum in these preglomerular parts that are composed of neurons with a similar morphology. Auditory toral fibers were not seen in the rostrolateral region of the PGl or PGlr, which clearly characterizes the region and confirms the heterogeneity of PGl pointed out based on nonexperimental materials in goldfish (Braford and Northcutt, 1983). Auditory toral terminals in the three auditory toral recipient parts of the PG share peculiar shapes. Each terminal appears like a donut or a pair of semicircles, presumably encircling a dendrite of the target preglomerular neuron to form a powerful synapse. The three auditory toral recipient parts of the PG also share reciprocal connections with the medial part of dorsal telencephalic area (Yamamoto and Ito, 2005; accompanying article). These preglomerular parts might in fact constitute a single neuronal population. Further experimental data on the connections of each part, however, are required to test this hypothesis.
Connections of the PG are apparently quite diverse among different teleosts, which may represent actual species differences or be caused by inconsistent terminologies. We thus discuss some major points concerning toral connections of the PG. As discussed above, the PGl of batrachoidids receives fibers from the auditory torus, while the PGl of Striedter (1992) or PGlr of the present study does not. Instead, PGl or PGlr in otophysans receives fibers from the lateral line portion of TS (Striedter, 1992; Yamamoto and Ito, unpubl. obs.). A preglomerular region receiving lateral line toral fibers may be lacking in batrachoidids (Weeg and Bass, 2000). In a percomorph rockfish, toral fibers terminate in a part of the PG (PGm of Yoshimoto et al., 1998), which is composed of a small-celled part and a medium-celled part (Murakami et al., 1986a). Specific injection experiments to the TSc and TSvl are required to clarify the toro-preglomerular connectional patterns in percomorphs. The organization and terminology of the PG in mormyrids appear quite distinct from those in cyprinids and acanthopterygians (Meek and Nieuwenhuys, 1998), and homology with other teleosts is quite difficult to establish at present. Clearly, detailed hodological data on the PG in various lineages are important to understand the evolution of this poorly understood complex and then to consider consistent terminology across lineages in the future.
Descending auditory pathways and their relay nuclei
The present study clarified various indirect descending pathways from diencephalic auditory centers via the torus and medial pretoral nucleus to the SO, which in turn projects to the DOm. These findings suggest that feedback control is important for auditory information processing in cyprinids. The significance of descending inputs on the rhombencephalic auditory neurons, in particular crest cells of the SO, is an interesting question to be studied.
Diencephalo-toral connections
The anterior tuberal nucleus and central posterior thalamic nucleus are the diencephalic sources to the cyprinid TSc (Echteler, 1984; present study). Both nuclei receive fibers from the TSc (Echteler, 1984; present study) as well as from the medial pretoral nucleus (present study) and thus presumably form auditory feedback circuits to the torus. Toropetal cells were found only in the central posterior thalamic nucleus in a catfish (Striedter, 1991) and in neither nuclei in batrachoidids (Bass et al., 2000, 2001) and mormyrid (Kozloski and Crawford, 1998), suggesting species differences in the diencephalo-toral connections.
In the present study the morphology of toropetal neurons in the central posterior thalamic nucleus was revealed in detail. They are quite large and possess three types of dendrites. The lateral dendrites are surrounded densely by torodiencephalic fibers and terminals, from which the dendrites may receive inputs. The medial dendrites extend to the smaller-celled portion of the central posterior nucleus and may receive inputs from torodiencephalic fibers terminating there or from other pathways. The rostral dendrites are very long. Surprisingly, the dendrites invade another auditory structure, the anterior tuberal nucleus. The rostral dendrites may receive inputs from torodiencephalic fibers terminating there or from other systems including intrinsic neurons. The dendritic morphology suggests integration of various types of auditory information by the large neurons.
Central nucleus of the torus (TSc)
Injections to the TSc labeled terminals in the SO, but tracer injections to the SO did not label TSc neurons in the present study. Injections involving the SO did not label neurons in the TSc in another study of goldfish (McCormick and Hernandez, 1996). The lack of labeled cells in the torus may be explained by failure to label existing toro-SO neurons or because TSc experiments led to labeling en-passant medial pretoral fibers to the SO. Auditory toral projections to the medial auditory nucleus, which includes secondary auditory neurons, is known in a different otophysan catfish (Finger and Tong, 1984).
The present toral injections also labeled terminals in the granule population, as reported in goldfish (McCormick and Hernandez, 1996). Echteler (1984) also refers to toral terminals in “granule cells immediately adjacent to the IVth ventricle” in carp, which most likely correspond to the granule population of McCormick and Hernandez (1996). This connection may be absent in a catfish (Ictalurus: Finger and Tong, 1984) but is present in a marine catfish (Arius: McCormick, 2001). It is unclear whether a homologous granule population is present in non-otophysans.
Medial pretoral nucleus
The medial pretoral nucleus was first described in catfish (Finger and Tong, 1984) and has been identified in other otophysans (Striedter, 1992; McCormick and Hernandez, 1996; present study) and in a batrachoidid (Bass et al., 2000). The nucleus is a periventricular nucleus in the mesodiencephalic boundary region, and has been sometimes regarded as a part of the torus (Striedter, 1991). This nucleus is connected reciprocally with the auditory torus (catfish: Finger and Tong, 1984; batrachoidid: Bass et al., 2000; cyprinids: present study) and with the SO (present study), indicating that the nucleus forms auditory feedback circuits to the mesencephalic and rhombencephalic auditory structures.
The present study suggests that the medial pretoral nucleus projects to the granule population and lateral part of the granular eminence, both of which in turn send fibers specifically to a part of the cerebellar crest overlying the SO. Thus, the pathways probably influence the activity of crest cells of the SO. Such descending pathways have not been reported in cyprinids or in other teleosts. Although our pretoral injections affected more ventral structures to some extent and reciprocal confirmation injections were not performed, the descending projections are likely efferents of the medial pretoral nucleus for two main reasons: 1) The medial pretoral nucleus appears an important origin of auditory feedback circuits, so it is not strange that the nucleus gives rise to descending pathways specific to the crest cells of the SO via different routes; 2) There appear to be no auditory structures ventral to the medial pretoral nucleus.
The medial pretoral nucleus receives afferents from the central posterior thalamic nucleus and anterior tuberal nucleus and projects to the ventral region of PGlr in otophysans (Striedter, 1991; present study). The medial pretoral nucleus appears reciprocally connected with the perilemniscular nucleus and may also project to the PGa, the medial zone of PGm, and PGlc in cyprinids (present study). The precise connectional pattern of medial pretoral nucleus, however, must be determined by more specific injections and reciprocal confirmation experiments.
Granule population and granular eminence
These populations of granular neurons presumably relay descending pretoral inputs to the crest cells of SO, as discussed above. The granule population corresponds to granule cells associated with medial auditory nucleus of the catfish, which became labeled after tracer injections to the medial auditory nucleus (Finger and Tong, 1984), probably due to involvement of the medial cerebellar crest in tracer injections.
Presumed pretoral fibers in these populations of granular neurons form large terminals with peculiar morphologies, the significance of which remains to be determined. Only a restricted lateral region of the granular eminence receives pretoral fibers, where descending fibers originate at the part of the cerebellar crest overlying the SO. The lateral region of the eminence thus may be specialized for the control of crest cells of the SO among several types of crest cells in the rhombencephalic octavolateral area. Labeled terminals were also seen in the granule population after tracer injections to the SO, and this population of granular cells may also receive ascending auditory fibers.
Acknowledgements
We thank Dr. Kosuke Imura (RIKEN, Wako, Japan) for help analyzing terminal distribution patterns in the torus, and Ms. Kyoko Fukushima and Mr. Kosuke Matsumoto for technical support in tissue processing.




