Cellular distribution and subcellular localization of molecular components of vesicular transmitter release in horizontal cells of rabbit retina†
This article is a US Government work and, as such, is in the public domain in the United States of America.
Abstract
The mechanism underlying transmitter release from retinal horizontal cells is poorly understood. We investigated the possibility of vesicular transmitter release from mammalian horizontal cells by examining the expression of synaptic proteins that participate in vesicular transmitter release at chemical synapses. Using immunocytochemistry, we evaluated the cellular and subcellular distribution of complexin I/II, syntaxin-1, and synapsin I in rabbit retina. Strong labeling for complexin I/II, proteins that regulate a late step in vesicular transmitter release, was found in both synaptic layers of the retina, and in somata of A- and B-type horizontal cells, of γ-aminobutyric acid (GABA)- and glycinergic amacrine cells, and of ganglion cells. Immunoelectron microscopy demonstrated the presence of complexin I/II in horizontal cell processes postsynaptic to rod and cone ribbon synapses. Syntaxin-1, a core protein of the soluble N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) complex known to bind to complexin, and synapsin I, a synaptic vesicle-associated protein involved in the Ca2+-dependent recruitment of synaptic vesicles for transmitter release, were also present in the horizontal cells and their processes at photoreceptor synapses. Photoreceptors and bipolar cells did not express any of these proteins at their axon terminals. The presence of complexin I/II, syntaxin-1, and synapsin I in rabbit horizontal cell processes and tips suggests that a vesicular mechanism may underlie transmitter release from mammalian horizontal cells. J. Comp. Neurol. 488:70–81, 2005. Published 2005 Wiley-Liss, Inc.
Visual processing begins at the photoreceptor synapse, known as the synaptic triad, which consists of the photoreceptor terminal, bipolar cell dendrites, and horizontal cell endings. Photoreceptors and bipolar cells form part of the direct pathway through the retina, whereas the horizontal cells serve as the principal interneurons of the outer retina. Horizontal cells are characterized by laterally distributed dendritic and axonal processes, which transmit a feedback signal to multiple photoreceptor axon terminals and a feedforward signal to bipolar cell dendrites (Baylor et al.,1971; Burkhardt,1993; Wu,1994). Although there is general agreement that horizontal cells mediate inhibitory feedback in the outer retina (Baylor et al.,1971; Mangel,1991), the nature of how these cells signal to their postsynaptic partners in the mammalian retina has remained poorly understood.
Conventional synaptic transmission in the central nervous system relies on the vesicular release of neurotransmitter, a set of coordinated steps that can be broken down into the docking, priming, and membrane fusion of synaptic vesicles (Südhof,2004). The N-ethylmaleimide-sensitive-factor attachment protein receptor (SNARE) complex is composed of three proteins, synaptosome-associated protein of 25 kD (SNAP-25), syntaxin, and synaptobrevin (also known as VAMP); it forms the molecular core that brings the vesicle in close apposition to the plasma membrane, leading to fusion. In addition to the proteins that make up the exocytotic core complex, progression through the synaptic vesicle cycle is directed and controlled by a number of key synaptic proteins, among them complexins, syntaxins, and synapsins (Südhof,2004).
Complexins are a set of small (18–21 kDa), highly charged, cytosolic proteins that bind to the fully formed exocytotic core complex at a late step in synaptic vesicle release (McMahon et al.,1995; Reim et al.,2001; Chen et al.,2002; Pabst et al.,2002) to regulate the Ca2+-dependent triggering of transmitter exocytosis (Reim et al.,2001; Archer et al.,2002). It is thought that complexins do so by binding and stabilizing the open conformation of syntaxin in the SNARE complex (Pabst et al.,2002; Chen et al.,2002; Archer et al.,2002). Synapsins are a family of abundant synaptic vesicle-associated proteins, involved in the calcium-dependent recruitment of synaptic vesicles (De Camilli et al.,1990). In the retina, synapsins are distributed to cells forming conventional synapses, but not ribbon synapses (Mandell et al.,1990,1992).
The vesicular γ-aminobutyric acid (GABA) transporter (VGAT) packages the neurotransmitter into synaptic vesicles (McIntire et al.,1997; Sagné et al.,1997). VGAT is strongly expressed in mammalian horizontal cells (Haverkamp et al.,2000; Cueva et al.,2002; Jellali et al.,2002), consistent with earlier findings of GAD expression in horizontal cells and a transmitter role for GABA. The highest level of VGAT immunostaining is in horizontal cell processes underneath the photoreceptor terminals, and in the dendritic and axonal endings within the synaptic triad (Haverkamp et al.,2000; Cueva et al.,2002; Jellali et al.,2002). Furthermore, the presence of small, clear-core vesicles in the horizontal cell processes and endings at photoreceptor synapses (Dowling et al.,1966; Fisher and Boycott,1974; Kolb,1977; Brandstätter et al.,1996) indicates that the morphological substrate for vesicular release is also present in these cell processes.
The proposed vesicular mechanism differs from the previously established plasmalemmal GABA transporter (GAT) mechanism, described for nonmammalian horizontal cells (Schwartz,2002). However, GAT isoforms do not localize to horizontal cells in rodent and rabbit retina (Brecha and Weigmann,1994, Johnson et al.,1996; Hu et al.,1999), and GABA uptake by adult mammalian horizontal cells has not been reported (Ehinger,1977; Blanks and Roffler-Tarlov,1982; Mosinger and Yazulla,1985; Wässle and Chun,1988,1989; Löhrke et al.,1994). Moreover, electrophysiological analyses of GABA-induced currents in isolated horizontal cells from rabbit and mouse retina have not revealed a transporter current (Blanco et al.,1996; Feigenspan and Weiler,2004).
To investigate the possibility that a vesicular mechanism mediates transmitter release from mammalian horizontal cells, the cellular distribution and subcellular localization of three presynaptic proteins were studied by immunocytochemistry in the rabbit retina. Localization of complexin I/II and its binding partner syntaxin-1, and of the synaptic vesicle-associated protein synapsin I to the tips of horizontal cell processes provides molecular support for the idea that mammalian horizontal cells utilize a vesicular mechanism of transmitter release.
MATERIAL AND METHODS
Adult New Zealand white rabbits of both sexes (n = 4, 3–4 kg, Charles River, Wilmington, MA) were used for these studies. All experiments were performed in accordance with the guidelines for the welfare of experimental animals issued by the Federal Governments of the United States and of Germany and were approved by the institutional animal use committees. The rabbits were initially administered ketamine and xylazine (Rompun; Bayer, Leverkusen, Germany; 0.25 mg/kg ketamine, 0.1 mg/kg Rompun, i.m.) followed by an overdose of sodium pentobarbital (200–400 mg/kg Nembutal i.v.; Merial, Hallbergmoos, Germany). The eyecups were fixed for 15–30 minutes in 4% paraformaldehyde (PFA) or 2% PLP (2% PFA, 1.37% D,L-lysine, 0.214% NaIO4) in 0.1 M phosphate buffer (PB), pH 7.4, for 20 minutes, cryoprotected in graded sucrose (10, 20, and 30%), and sectioned vertically at 12–14 μm on a cryostat. For pre-embedding electron microscopy, retinas were fixed for 50 minutes in 4% PFA.
Antibodies
Primary antibodies and their dilutions were as follows: rabbit polyclonal antibodies against complexin (CPX)-I/II (1:1,000; Synaptic Systems, Göttingen, Germany, #122 002, antiserum #1 raised against a conserved peptide [peptide #1, amino acids 122–134, KYLPGPLQDMFKK] and 1:2,000–10,000, #122 103, affinity-purified antibody against peptide #2 from CPX II [peptide #2, amino acids 45–81, EEERKAKHARMEAEREKVRQQIRDKYGLKKKEEKEAE] but recognizes both CPX I & II; Reim et al.,2001; Table 1). The CPX I/II antibodies recognized bands of the correct molecular weight on Western blots, and the bands could be selectively blocked by the antigenic peptide (Synaptic Systems product sheet). Preabsorption of the CPX I/II antiserum #1 with 1 μM peptide #1 resulted in no specific immunolabeling; whereas, CPX I/II antiserum #2 immunolabeling was not blocked by peptide #1 (data not shown). The HPC-1 mouse monoclonal antibody against syntaxin-1 (1:2,500; Sigma, St. Louis, MO; S0664; Barnstable et al.,1985) recognized a band of the correct molecular weight on Western blots of rat retinal proteins (Inoue et al.,1992). Two antibodies to synapsin I were used: a rabbit polyclonal antibody (1:500–1:2,000; P610; DeCamilli et al.,1983; Geppert et al.,1994; von Kriegstein et al.,1999) and a mouse monoclonal antibody (1:100; Chemicon, Temecula, CA; MAB355; Smith et al.,1993). The specificity of both has been demonstrated on Western blots, and preabsorption of the antibody with purified synapsin I resulted in a block of immunolabeling (De Camilli et al.,1983; Smith et al.,1993). In addition, double-label experiments with the two synapsin I antibodies produced a complete co-localization of immunoreactivity (data not shown). Various antibodies to calbindin D-28k have been used to immunostain horizontal cells in mammalian retinae (Röhrenbeck et al.,1987; Lyser et al.,1994; Peichl and González-Soriano,1994; Massey and Mills,1996; Johnson and Vardi,1998; Haverkamp and Wässle,2000; Völgyi and Bloomfield,2002; Raven and Reese,2003; Loeliger and Rees,2005). The labeling patterns of horizontal cells in rabbit retina produced were similar with the mouse and rabbit calbindin antibodies that were used: mouse monoclonal antibody against calbindin (1:2,500–5,000; Sigma; C9848, clone CB-955; Celio et al.,1990) and rabbit polyclonal antibody against calbindin (1:1,000; Swant, Bellinzona, Switzerland; CB38; Garcia-Segura et al.,1984).
| Antiserum | Host | Immunogen | Source | Working dilution |
|---|---|---|---|---|
| Complexin I/II | Rabbit | Complexin I/II a.a. 122–134 | Synaptic Systems, Göttingen, Germany, #122 002 (Reim et al.,2001) | 1:1,000–1:2,000 |
| Complexin I/II | Rabbit | Complexin II a.a. 45–81 | Synaptic Systems, #122 103 (Reim et al.,2001) | 1:2,000–1:10,000 |
| Calbindin D-28k | Rabbit | Chicken gut calbindin D-28k | Swant, Bellinzona, Switzerland, CB38 (Celio et al.,1990) | 1:1,000 |
| Calbindin D-28k | Mouse | Purified bovine kidney calbindin D-28k | Sigma, St. Louis, MO, C9848, clone CB955 (Garcia-Segura et al.,1984) | 1:2,500–1:5,000 |
| GABA | Rat | GABA conjugated to thyroglobulin w/PFA | D. Pow, Brisbane, Australia (Pow et al.,1995) | 1:200 |
| Glycine | Rat | Glycine conjugated to thyroglobulin w/PFA | D. Pow (Pow et al.,1995) | 1:1,000 |
| Syntaxin-1/HPC-1 | Mouse | Hippocampal plasma membranes (HPC) | Sigma, S0664 (Barnstable et al.,1985; Inoue et al.,1992) | 1:2,500 |
| Synapsin 1 | Rabbit | Purified bovine synapsin 1 | H. Betz, Frankfurt, Germany (De Camilli et al.,1983; Geppert et al.,1994; von Kriegstein et al.,1999) | 1:500–2,000 |
| Synapsin 1 | Mouse | Purified sheep brain synapsin 1 | Chemicon, Temecula, CA, MAB355 (Smith et al.,1993) | 1:100 |
| VGLUT1 | Guinea pig | Synthetic peptide from rat VGLUT1 | Chemicon, AB5905 (Todd et al.,2003; Sherry et al.,2003a; Fyk-Kolodziej et al.,2004) | 1:20,000 |
- GABA, γ-aminobutyric acid; VGLUT1, vesicular glutamate transporter 1; a.a., amino acids; PFA, paraformaldehyde.
To identify the two major classes of amacrine cells, antibodies raised against GABA and glycine conjugated to thyroglobulin with PFA (Pow et al.,1995) were employed: a rat polyclonal antibody to GABA (1:200; David Pow, Brisbane, Australia; Pow et al.,1995) and a rat polyclonal antibody to glycine (1:1,000; D. Pow; Pow et al.,1995). The specificity of these antibodies was established on dot blots against five related amino acids (Pow et al.,1995). A guinea pig polyclonal antibody against VGLUT1 (1:20,000; Chemicon; AB5905) was used to identify photoreceptor and bipolar cell axon terminals (Johnson et al.,2003; Sherry et al.,2003a; Fyk-Kolodziej et al.,2004). The staining pattern obtained with the guinea pig VGLUT1 antibody corresponds to that described by other VGLUT1 antisera (Todd et al.,2003), and preabsorption of the VGLUT1 antiserum with the antigenic peptide results in a block of specific immunostaining (Fyk-Kolodziej et al.,2004). The labeling of the primary antibodies was visualized by using fluorochrome-conjugated secondary antibodies: Alexa 488, and Alexa 568 or Alexa 594 goat-anti-rabbit IgG, goat anti-mouse IgG, goat anti-rat IgG, or goat anti-guinea pig IgG (Molecular Probes, Eugene, OR) used at 1:500.
Immunohistochemistry
Light microscopy.
Immunohistochemical labeling was carried out by using the indirect immunofluorescence method. Cryostat sections were incubated in 10% normal goat serum (NGS), 1% bovine serum albumin (BSA), 0.5% Triton X-100, 0.05% sodium azide in 0.01 M phosphate-buffered saline (PBS), pH 7.4, for 1 hour. The blocking solution was replaced by the primary antibody diluted in 3% NGS, 1% BSA, 0.5% Triton X-100, 0.05% sodium azide in PBS and incubated overnight at room temperature in a humidified chamber. Following washes in 0.01 M PBS or 0.1 M phosphate buffer (PB, pH 7.4), the sections were incubated for 40–60 minutes in secondary antibody mix, diluted in the same solution as the primary, except that sodium azide was omitted. Following washes in 0.01 M PBS or 0.1 M PB, the sections were mounted in Aqua Poly/Mount (Polysciences, Warrington, PA) or Prolong Antifade (Molecular Probes, Eugene, OR). Double-immunolabeling experiments were performed by incubating the sections in a mixture of antibodies followed by a cocktail of secondary antibodies. Control sections were processed excluding the primary antibodies, resulting in no specific labeling. For double-label experiments, primary antibodies were run individually to confirm labeling patterns.
The sections were examined and analyzed on a Zeiss LSM 510 Meta or a Pascal confocal microscope (Zeiss, Oberkochen, Germany) equipped with argon (Ar) and helium/neon (He/Ne) lasers, using a 40× 1.3 n.a. Plan-Neofluar or a 63× 1.25 n.a. Plan-Neofluar objective and the appropriate fluorescence filter sets. Images were taken at a resolution of 2,048 × 2,048 pixels. Brightness and contrast of digital images were adjusted by using Adobe Photoshop 5.0 (Adobe Systems, Inc., San Jose, CA). Projections of three to five optical slices (0.5–1.0 μm/slice) are shown, in order to capture the winding horizontal cell processes.
Pre-embedding immunoelectron microscopy.
Immunolabeling was performed on rabbit retina by using pre-embedding immunoelectron microscopy techniques, as previously described (Brandstätter et al.,2004). In brief, the eyecups were fixed for 50 minutes in 4% PFA. The retinae were dissected, cryoprotected in graded sucrose, and frozen and thawed three times to improve antibody penetration. Vibratome sections were cut at 50 μm into cold PBS, pH 7.4. The primary antibodies were used in the same concentration and diluted in the same media used for light microscopy, except that Triton X-100 was omitted, and the sections were incubated for 4 days at 4°C. The immunolabeling was visualized by the ABC method (Vectastain Elite ABC kit, Vector, Burlingame, CA) using 3,3′-diaminobenzidine (DAB) as substrate. The DAB reaction product was silver intensified and gold toned, and the tissue was flat embedded in Epon 812 (Serva, Heidelberg, Germany). Ultrathin sections were cut and then contrasted with uranyl acetate and lead citrate. The ultrathin sections were examined and photographed with a Zeiss EM10 electron microscope and a GATAN BioScan digital camera (1,024 × 1,024 pixel; GATAN, Munich, Germany) in combination with the software program DIGITAL MICROGRAPH 3.1 (GATAN, Pleasanton, CA).
RESULTS
Complexin I/II
Antibodies to complexin I/II (CPX I/II) labeled a variety of cell types, the two plexiform layers, and the nerve fiber layer in rabbit retina (Fig. 1A). There was no superior to inferior gradient across the retina of CPX I/II immunoreactivity. There was no labeling of photoreceptor cell bodies in the outer nuclear layer or of bipolar cell bodies in the inner nuclear layer (INL). Cell bodies of horizontal cells and of subpopulations of amacrine and ganglion cells were prominently labeled. Large, strongly CPX I/II-immunoreactive cell bodies with thick processes were observed in the ganglion cell layer and the nerve fiber layer (Fig. 1A). Due to the highly conserved amino acid sequence of the two CPX isoforms (McMahon et al.,1995), it is not possible to determine whether CPX I or CPX II, or both, are present in the labeled cells. There was no change in labeling pattern between the two different fixations used, nor did the pattern of labeling differ when the antiserum and affinity-purified peptide antibodies were compared, except that a background labeling of Müller cells was missing when the affinity-purified antibody was used. All CPX I/II figures shown were done with the affinity-purified CPX I/II antibody #2. Thirty-minute fixation with 4% PFA appeared to produce slightly stronger labeling and less background than the shorter 15-minute fixation.
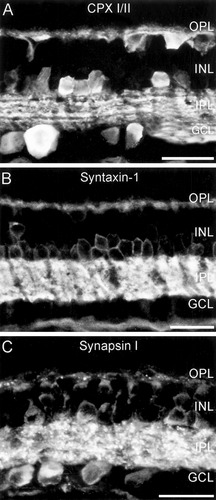
Fluorescence micrographs of labeling produced by antibodies to (A) complexin I/II, (B) syntaxin-1, and (C) synapsin I (P610) in vertical sections of rabbit retina. Note the prominent immunoreactivity in the plexiform layers for all three synaptic proteins. OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 20 μm in A–C.
CPX I/II immunoreactivity spanned the entire inner plexiform layer (IPL), with the highest concentration of immunolabeled processes in the middle of the IPL (30–70% depth), indicating that projections to both OFF and ON sublaminae contained CPX I/II immunoreactivity. Strata of sparser CPX I/II immunoreactivity flanked the central CPX I/II-dense layer. In the outer plexiform layer (OPL), labeled processes of horizontal cells with endings projecting in the sclerad direction were observed.
Syntaxin-1
To establish whether binding partners of complexins were present (Tokumaru et al.,2001), the distribution of syntaxin-1 was determined in rabbit retina. Syntaxin-1 immunoreactivity was robust in the IPL and outlined numerous amacrine cell bodies in the proximal INL (Fig. 1B), as previously described for rat retina (Barnstable et al.,1985; Morgans et al.,1996). In addition to the prominent IPL labeling, there was distinct immunoreactivity in horizontal cells and their processes in the OPL (Fig. 1B).
Synapsin I
As synapsins were shown to be markers for conventional synapses in retina (Mandell et al.,1990), we next examined the distribution of synapsin I (Fig. 1C). The rabbit polyclonal and mouse monoclonal antibodies produced identical labeling patterns (data not shown). Numerous cell bodies were immunolabeled, including those of horizontal, bipolar, amacrine, and ganglion cells. Intense synapsin I immunoreactivity was present throughout the IPL, and sparse punctate labeling could be seen in the OPL. Evaluation of the OPL at high magnifications showed that synapsin I immunoreactivity was localized to dendrites of bipolar cells and to the processes and endings of horizontal cells. In contrast, synapsin I was absent from bipolar cell axon terminals, as previously described (Mandell et al.,1990) and as determined by double labeling with VGLUT1 as a marker of bipolar cell terminals (data not shown; Johnson et al.,2003; Sherry et al.,2003a; Fyk-Kolodziej et al.,2004).
Complexin I/II immunoreactivity localizes to horizontal cells
CPX I/II immunoreactivity appeared to localize exclusively to horizontal cells and their processes in the outer retina, as photoreceptors and bipolar cells did not express CPX I/II. To confirm this CPX I/II localization, retina sections were double labeled with antibodies to CPX I/II and calbindin, a marker for horizontal cells (Fig. 2; Röhrenbeck et al.,1987; Massey and Mills,1996; Mitchell et al.,1995). Calbindin antibodies identify both types of horizontal cells in rabbit retina (Röhrenbeck et al.,1987; Massey and Mills,1996; Mitchell et al.,1995), with type A cells exhibiting greater immunoreactivity than the type B cells (Röhrenbeck et al.,1987; Lyser et al.,1994; Massey and Mills,1996). The complete co-localization of CPX I/II (red) and calbindin (green) indicated that all horizontal cells expressed CPX I/II (Fig. 2A–C). Note that the entire horizontal cell, including the cell body, processes, and distal protuberances, contained CPX I/II immunoreactivity. To confirm the subcellular localization of CPX I/II to horizontal cell endings at photoreceptor synapses, pre-embedding electron microscopy experiments were conducted. They revealed strong immunolabeling of the lateral elements, the processes of horizontal cells, at the ribbon synaptic sites in cone pedicles (Fig. 2D) and rod spherules (Fig. 2E,F). Sometimes only one and sometimes both or more than one of the lateral horizontal cell processes in rod and cone terminals, respectively, were labeled for CPX-I/II. Note that the surrounding photoreceptor terminals were free of immunolabel, as were the invaginating bipolar cell processes.
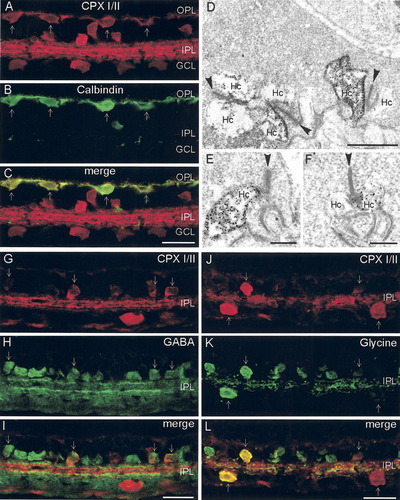
Localization of CPX I/II to horizontal cells and amacrine cells. A–C: Co-localization of (A) CPX I/II with (B) calbindin, a horizontal cell marker, showed clear double labeling (arrows) of somata and processes in the OPL (a stack of optical slices projected onto a single plane in order to visualize fully the thin horizontal cell process in the OPL; C). Pre-embedding immunoelectron microscopic labeling for CPX I/II shows dark granular DAB reaction product in horizontal cell processes at (D) cone and (E,F) rod photoreceptor synapses. Arrowheads point to the synaptic ribbons in the photoreceptor terminals.G–I: Double-label experiments with (G) CPX I/II and (H) GABA antibodies demonstrated the localization of CPX I/II to some GABAergic amacrine cell bodies (I, arrows). J–L: Double-label experiments with (J) CPX I/II and (K) glycine antibodies showed the localization of CPX I/II to some glycinergic amacrine cell bodies (L, arrows). Note that there are numerous amacrine cell bodies that do not express CPX I/II. OPL, outer plexiform layer; IPL, inner plexiform layer; GCL, ganglion cell layer; Hc, horizontal cell. Scale bar = 20 μm in C (applies to A–C); 20 μm in I (applies to G–I); 20 μm in L (applies to J–L); 1 μm in D; 0.3 μm in E,F.
CPX I/II is present in GABAergic and glycinergic amacrine cells
As syntaxin-1 and synapsin I are established as markers for amacrine cells in a number of species (Barnstable et al.,1985; Mandell et al.,1990,1992; Morgans et al.,1996), we focused on characterizing CPX I/II expression in the inner retina. There were somata in the proximal INL that exhibited intense as well as those that showed weak CPX I/II immunoreactivity (Fig. 2G,J). Interstitial amacrine cells were also labeled for CPX I/II (data not shown). To determine whether the CPX I/II-expressing cells segregated with either of the two major classes of amacrine cells, GABAergic or glycinergic, retina sections were double labeled for CPX I/II and the transmitters, GABA or glycine (Fig. 2G–I,J–L). GABAergic amacrine cells showed co-localization in subsets of strongly and weakly CPX I/II-immunoreactive cells (Fig. 2G–I, arrows). In the IPL, GABAergic processes appeared to stratify above and below the CPX I/II-rich middle layer (Fig. 2I). Similarly, populations of glycinergic amacrine cells showed strong and weak CPX I/II labeling (Fig. 2J–L). It appears, however, that the glycinergic processes stratify within the CPX I/II-rich middle layer in the IPL. Numerous GABAergic and glycinergic amacrine cells did not express CPX I/II (Fig. 2I,L). These results indicate that subpopulations of GABAergic and glycinergic amacrine cells in rabbit retina express CPX I/II, and that CPX I/II expression does not segregate with either of the major fast inhibitory transmitters in the rabbit inner retina.
Syntaxin-1 is present in horizontal cells
To determine whether the syntaxin-1 immunoreactivity in the OPL arose from horizontal cells, vertical sections were double labeled for syntaxin-1 and calbindin (Fig. 3A–C). Unlike CPX I/II, which filled the entirety of the horizontal cell, syntaxin-1 immunoreactivity occurred principally in the processes and endings of the horizontal cells (Fig. 3C). Outlines of cell bodies could also be observed, indicating membrane localization of the protein. Some amacrine cells also showed double labeling for calbindin and syntaxin-1 (Fig. 3C, arrows). Pre-embedding immunoelectron microscopy demonstrated strong labeling of horizontal cell processes at cone (Fig. 3D,E) and rod (Fig. 3F–H) photoreceptor ribbon synapses. Sometimes only one and sometimes both of the two lateral horizontal cell processes at the ribbon synaptic site were labeled for syntaxin-1 (Fig. 3D–H).
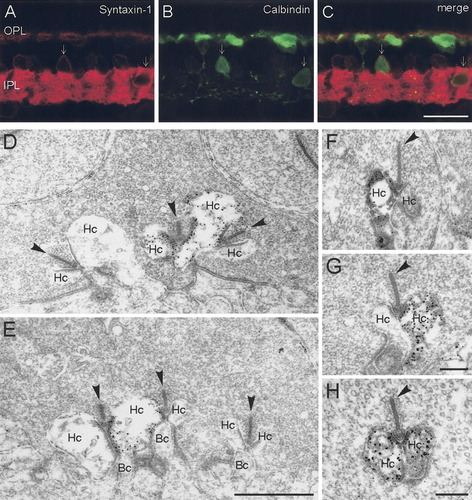
Cellular and subcellular localization of syntaxin-1 to horizontal cells and their processes and endings in photoreceptor synapses. (A) Syntaxin-1 (red) co-localized with (B) calbindin (green) in horizontal cell bodies and processes in the OPL, as well as some amacrine cell bodies (arrows) in the INL. In addition to horizontal cells, calbindin immunoreactivity was present in a subtype of bipolar cell and amacrine cells in the rabbit retina. Pre-embedding immunoelectron microscopy with the HPC-1 monoclonal antibody produced dark, granular DAB immunolabeling for syntaxin-1 in horizontal cell endings at (D,E) cone and (F–H) rod photoreceptor synapses. OPL, outer plexiform layer; IPL, inner plexiform layer; Hc, horizontal cell; Bc, bipolar cell. Arrowheads point to the synaptic ribbons in the photoreceptor terminals. Scale bar = 20 μm in C (applies to A–C; 1 μm in E (applies to D,E); 0.3 μm in G (applies to F,G); 0.3 μm in H.
CPX I/II, syntaxin-1, and synapsin I co-localize in the same horizontal cells
To establish that the synaptic proteins were expressed in the same cells, a series of double-label experiments were performed. Figure 4A shows a vertical section of rabbit retina double labeled for CPX I/II and syntaxin-1, with clear co-localization in horizontal and amacrine cells. Note the robust co-localization in the horizontal cell processes, extending into the fine endings, the presumed sites of contact with the photoreceptor terminals and bipolar cell dendrites (Fig. 4B–D, arrows). Double labeling for synapsin I and syntaxin-1 confirmed that both proteins are expressed in the same horizontal cells (Fig. 5A–C). The synapsin I immunolabeling in the OPL was quite weak, but pre-embedding immunoelectron microscopy verified subcellular localization of synapsin I to horizontal cell axonal endings at rod photoreceptor synapses (Fig. 5D,E) and occasional bipolar cell dendrites (data not shown). The low intensity of labeling in the OPL makes the unequivocal determination of the pattern of synapsin I expression in the retina difficult. Although the rather weak labeling for synapsin I in some horizontal cells suggests that there may be subpopulations of synapsin-I-expressing cells, we cannot rule out that it may simply reflect a threshold of detectability of the signal.
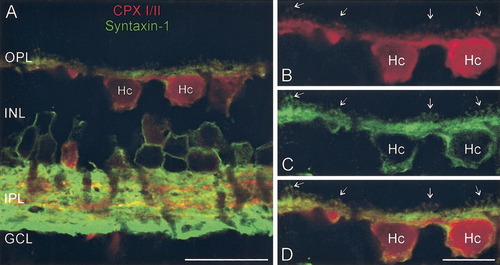
CPX I/II and syntaxin-1 are expressed in the same horizontal cells. A: Vertical section of rabbit retina stained for CPX I/II (red) and syntaxin-1 (green). Note the co-localization in horizontal and amacrine cell bodies. B–D: Higher magnification view of horizontal cells shows the co-localization of CPX I/II (B) and syntaxin-1 (C) in processes and endings (arrows) of horizontal cells (D). Note the membrane localization of syntaxin-1 to the plasma membrane of horizontal cells. OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; Hc, horizontal cell. Scale bar = 20 μm in A; 10 μm in D (applies to B–D).
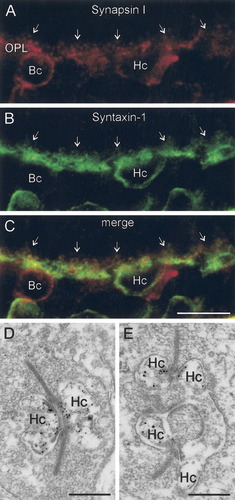
Synapsin I and syntaxin-1 co-localize in horizontal cells. A–C: Vertical section of rabbit retina stained for synapsin I (red) and syntaxin-1 (green). A: Synapsin I immunoreactivity is present in horizontal and bipolar cells. B: Syntaxin-1 co-localizes with synapsin I in horizontal cells (C). Note that the co-localization appears to extend into the protrusions coming off of horizontal cell processes (arrows). D,E: Electron micrographs of synapsin I immunoreactivity show localization to horizontal cell endings within rod photoreceptor terminals. OPL, outer plexiform layer; Hc, horizontal cell; Bc, bipolar cell. Scale bar = 10 μm in C (applies to A–C); 0.4 μm in D,E.
Taken together, the co-localization of complexin I/II, syntaxin-1 and synapsin I to horizontal cell endings within photoreceptor ribbon synapses indicates that these cells contain components of the molecular machinery required for regulated vesicular release of neurotransmitter.
DISCUSSION
Distribution of synaptic proteins in the mammalian retina
This study demonstrates the co-expression of the synaptic proteins, CPX I/II, syntaxin-1, and synapsin I, in horizontal cells of the mammalian retina. The cellular distribution of CPX I/II to horizontal, amacrine and ganglion cells in rabbit retina is similar to that observed in mouse retina (Brandstätter et al.,2003), suggesting a shared mammalian pattern of expression. In addition to the prominent immunolabeling of CPX I/II in the synaptic layers of the retina, CPX I/II was observed in cell bodies, possibly due to the cytosolic nature of CPX I/II (McMahon et al.,1995). The binding partner of CPX I/II, syntaxin-1, is also present in horizontal cells, as is the synaptic vesicle-associated protein, synapsin I. Moreover, immunoelectron microscopy showed the presence of all three synaptic proteins in the lateral elements of horizontal cells at rod and cone photoreceptor synapses.
Emerging evidence has shown that conventional and ribbon synapses employ different isoforms of synaptic proteins, reflecting their different kinetics of transmitter release. Synapsins were the first synaptic proteins shown to exhibit a differential distribution between ribbon and conventional synapses in the retina (Mandell et al.,1990,1992). Synapsins are absent from ribbon synapses (Mandell et al.,1990,1992; Geppert et al.,1994; Ullrich and Südhof,1994; but see von Kriegstein et al.,1999), but they are a feature of most conventional synapses (Mandell et al.,1990,1992; De Camilli et al.,1990). Therefore, the presence of synapsin I in rabbit horizontal cells raises the possibility of conventional vesicular release from these cells, and it confirms the synapsin I mRNA localization to rat horizontal cells and its absence from photoreceptors (Haas et al.,1990). Interestingly, there was also an apparent exclusion of CPX I/II from ribbon synapses, including the photoreceptor terminals in the OPL, indicating that CPX I/II is utilized predominantly by conventional synapses in the rabbit retina. CPX I/II, however, does not account for all conventional synapses, as it is clear that not all amacrine and ganglion cells express CPX I/II. This finding suggests the likelihood that other isoforms of complexin are expressed in rabbit retina. For example, in mouse retina, glycinergic amacrine cells, bipolar cells, and photoreceptors express CPX III, whereas photoreceptors express also CPX IV (Brandstätter et al.,2003). Finally, syntaxin-1 expression characterizes conventional synapses, whereas a different isoform, syntaxin 3, localizes to ribbon synapses (Morgans et al.,1996).
Transmitter release from horizontal cells
GABA is likely to be a transmitter of mammalian horizontal cells (Schwartz,2002; Yang,2004). One or both isoforms of the synthetic enzyme L-glutamate decarboxylase (GAD) are found in the horizontal cells at the mRNA (Sarthy and Fu,1989) and protein levels (Vardi et al.,1994; Vardi and Auerbach,1995; Johnson and Vardi,1998). Many, but not all, studies have shown GABA immunoreactivity in horizontal cells of cat, rabbit, and primate retina (Mosinger et al.,1986; Mosinger and Yazulla,1987; Wässle and Chun,1989; Pourcho and Owczarzak,1989; Grünert and Wässle,1990; Kalloniatis et al.,1996; Johnson and Vardi,1998). In addition, several studies report low or nondetectable levels of GAD67 or GABA immunoreactivity in horizontal cells of the adult rabbit, mouse, and rat retinae (Brandon,1985; Brecha et al.,1991; Yazulla et al.,1997; Johnson and Vardi,1998; Koulen et al.,1998); however, GAD immunostaining is present at high levels in horizontal cells of the developing and juvenile mouse retina (Schnitzer and Rusoff,1984). The detection of GAD or GABA in the adult retina can be influenced by several factors, including differential expression of GAD isoforms, differing levels of GAD and GABA in horizontal cells, and technical issues related to fixation protocols and antibody specificity (Vardi et al.,1994; Vardi and Auerbach,1995; Kalloniatis et al.,1996; Johnson and Vardi,1998). Finally, GABA receptors are found postsynaptically in the outer plexiform layer on cone photoreceptor terminals and shafts of bipolar cell dendrites (Vardi et al.,1998; Wässle et al.,1998).
The supposition that rabbit horizontal cells use a vesicular mode of transmitter release was prompted by the immunolocalization of VGAT, a synaptic vesicle protein necessary for loading inhibitory transmitter into synaptic vesicles (McIntire et al.,1997) to these cells (Haverkamp et al.,2000; Cueva et al.,2002; Jellali et al.,2002). Indeed, the highest level of VGAT immunoreactivity occurs in horizontal cell processes underneath the photoreceptor terminals and in the dendritic and axonal endings within the synaptic triad (Haverkamp et al.,2000; Cueva et al.,2002). The localization of synaptic proteins involved in regulated transmitter release, such as complexin I/II, syntaxin-1, and synapsin I, to rabbit horizontal cells provides further molecular support for a vesicular release mechanism. Moreover, other synaptic proteins have been localized to the OPL and horizontal cells in the mammalian retina, including synaptoporin (synaptophysin II) (Brandstätter et al.,1996) and SNAP-25 (Catsicas et al.,1992; Ullrich and Südhof,1994; Grabs et al.,1996; von Kriegstein et al.,1999; West Greenlee et al.,2001). At present, the synaptobrevin (VAMP) isoform, the third component of the SNARE complex, which is present in mammalian horizontal cells, has not yet been identified, although those typically found synaptically do not appear to be present (Sherry et al.,2003b).
The growing evidence for vesicular transmitter release by mammalian horizontal cells counters the conventional view from work in toad and fish retina (Schwartz,1982,1987,2002) that GABA is released from horizontal cells via the reverse transport of GABA by a plasma membrane GABA transporter. To date, there is no support for a transporter-mediated mechanism in mammalian horizontal cells. For example, GATs have not been localized to rodent and rabbit horizontal cells (Brecha and Weigmann,1994; Johnson et al.,1996; Hu et al.,1999), and patch-clamp recordings of isolated mammalian (rabbit and mouse) horizontal cells have not revealed the presence of GABA-dependent transporter currents (Blanco et al.,1996; Feigenspan and Weiler,2004). Finally, no high-affinity transport of [3H] GABA or GABA analogs by horizontal cells has been reported for any adult mammalian retina (Ehinger,1977; Blanks & Roffler-Tarlov,1982; Mosinger and Yazulla,1985; Wässle & Chun,1988,1989; Löhrke et al.,1994). More recently, alternative mechanisms of horizontal cell feedback involving electrical resistance or ephaptic feedback onto cones in goldfish (Kamermans et al.,2001; Kamermans and Fahrenfort,2004) and a pH-dependent modulation of cone calcium channels in newts (Hirasawa and Kaneko,2003) have been proposed. Proton release that occurs with vesicular release may contribute to an increase in proton concentration in the synaptic cleft that produces the shift in the current-voltage relation of the calcium current (Hirasawa and Kaneko,2003). Finally, horizontal cells may utilize multiple feedback mechanisms.
Synaptic transmission from horizontal cell processes: axons and dendrites
Unlike complexin I/II immunoreactivity, immunolabeling for syntaxin-1 and synapsin I showed a more restricted subcellular localization, namely to the processes and endings of horizontal cells, suggesting that vesicular transmitter release may occur from these cellular compartments. The ultrastructural localization of all three proteins placed them in horizontal cell endings invaginating photoreceptor synaptic triads, where the intercellular interactions are thought to occur, as expected for synaptic proteins that are involved in transmitter release. In electron micrographs of well-preserved horizontal cell endings, vesicles are clearly present (Fisher and Boycott,1974; Kolb,1977; R.F. Dacheux and H. Wässle, personal communications), albeit fewer in number than in photoreceptor ribbon synapses. Furthermore, previous electron microscopic data (Brandstätter et al.,1996) on synaptoporin localization demonstrated the presence of vesicle clusters within rabbit horizontal cell processes. Although it remains to be proven that they are synaptic vesicles, these observations suggest that the morphological substrate for vesicular release is present in horizontal cell endings and processes. Moreover, it is likely that the reduced intensity of immunolabeling of synaptic vesicle-associated proteins in horizontal cells, compared with the synapses in the IPL, reflects this lower density of synaptic vesicles.
Type B horizontal cells innervate rod terminals with their axonal endings and cone terminals with their dendrites, whereas the axonless type A horizontal cells innervate only cone terminals (Peichl et al.,1998). The presence of CPX I/II and syntaxin-1 at both rod and cone synapses and the strong immunolabeling of horizontal processes beneath the terminals suggest that transmitter release probably occurs from both dendritic and axonal processes of horizontal cells. Dendritic release of GABA occurs in a calcium-dependent manner in other systems, such as the olfactory bulb and thalamus (Isaacson,2001; Munsch et al.,2003). Dendritic release shares many properties with the regulated exocytosis that occurs at axon terminals, including sensitivity to tetanus toxin and the presence of synaptic proteins involved in regulated exocytosis, although the isoforms may differ from those used at the axon terminals (Maletic-Savatic and Malinow,1998; Ludwig and Pittman,2003).
Targets for GABA release
The relative lack of pre- and postsynaptic specializations within photoreceptor synapses makes the determination of GABA targets difficult (Yazulla,1995; Linberg and Fisher,1998); nevertheless, there are data to suggest that all three cell types involved in the triad synapse may be targets. Mammalian horizontal cells express GABAA receptors (Greferath et al.,1994,1995) and exhibit GABA-induced currents (Blanco et al.,1996; Feigenspan and Weiler,2004), suggesting that GABA can act presynaptically at an autoreceptor. The localization of GABA receptors to both cone terminals and bipolar cell dendrites is consistent with both a feedback and a feedforward role for GABA released from horizontal cells. Cone terminals of pig, mouse, and rat show GABAC receptor (ρ subunit) immunoreactivity (Picaud et al.,1998; Pattnaik et al.,2000). In support of this hypothesis, recordings from mouse and pig cones showed the presence of functional GABAA and GABAC receptors (Pattnaik et al.,2000), whereas rods in porcine retina exhibited little sensitivity to GABA (Picaud et al.,1998).
GABA receptors have been found on bipolar cell dendrites (GABAA: Greferath et al.,1994; Vardi and Sterling,1994; Vardi et al.,1998; GABAC: Enz et al.,1996; Haverkamp et al.,2000; Pattnaik et al.,2000), suggesting a mechanism for a feedforward process. GABAA receptor immunoreactivity is localized to bipolar cell membranes adjacent to horizontal cell endings in cone pedicles and underneath photoreceptor terminals (Vardi and Sterling,1994; Haverkamp et al.,2000). In monkey retina, GABA receptors are sandwiched between layers of horizontal cell processes underneath cone pedicles (Haverkamp et al.,2000), suggesting that the action of GABA might be localized to the general region, if not strictly to a given synapse (Wässle et al.,2003).
Antagonistic receptive field surrounds can be detected in mammalian bipolar cells (Dacey et al.,2000). GABA released from horizontal cells onto bipolar cell dendrites could participate in this process (Yang,2004). GABA elicits depolarizing inward currents when applied to dendrites of mouse rod bipolar cells and hyperpolarizing currents when applied to those of OFF-bipolar cells, suggestive of feedforward input from horizontal cells (Satoh et al.,2001). Differential chloride concentrations in the dendrites would be one mechanism for generating surrounds of opposite polarity, as suggested by the differential expression of chloride transporters in bipolar cell dendrites (Vardi et al.,2000). Taken together, these studies indicate that there are multiple targets for GABA in the OPL, which could mediate the responses of GABA released from horizontal cells in the retina.
In summary, the presence of synaptic proteins, complexin I/II, syntaxin-1, and synapsin I, in the processes of mammalian horizontal cells at photoreceptor synapses provides molecular support for the regulated vesicular release of transmitter from these cells.
Acknowledgements
We gratefully acknowledge the excellent technical support of Anja Staab, Alejandro Vila, and Rebecca Jones. We thank Drs. H. Wässle, I. D'Angelo, S. Stella, and J. Sinclair for critically reading and improving the article.




