Central projections of the saccular and utricular nerves in macaques
Abstract
The central projections of the utricular and saccular nerve in macaques were examined using transganglionic labeling of vestibular afferent neurons. In these experiments, biotinylated dextran amine was injected directly into the saccular or utricular neuroepithelium of fascicularis (Macaca fascicularis) or rhesus (Macaca mulatta) monkeys. Two to 5 weeks later, the animals were killed and the peripheral vestibular sensory organs, brainstem, and cerebellum were collected for analysis. The principal brainstem areas of saccular nerve termination were lateral, particularly the spinal vestibular nucleus, the lateral portion of the superior vestibular nucleus, ventral nucleus y, the external cuneate nucleus, and cell group l. The principal cerebellar projection was to the uvula with a less dense projection to the nodulus. Principle brainstem areas of termination of the utricular nerve were the lateral/dorsal medial vestibular nucleus, ventral and lateral portions of the superior vestibular nucleus, and rostral portion of the spinal vestibular nucleus. In the cerebellum, a strong projection was observed to the nodulus and weak projections were present in the flocculus, ventral paraflocculus, bilateral fastigial nuclei, and uvula. Although there is extensive overlap of saccular and utricular projections, saccular inputs to the lateral portions of the vestibular nuclear complex suggest that saccular afferents contribute to the vestibulospinal system. In contrast, the utricular nerve projects more rostrally into areas of known concentration of vestibulo-ocular related cells. Although sparse, the projections of the utricle to the flocculus/ventral paraflocculus suggest a potential convergence with floccular projection inputs from the vestibular brainstem that have been implicated in vestibulo-ocular motor learning. J. Comp. Neurol. 466:31–47, 2003. © 2003 Wiley-Liss, Inc.
The bilateral otolith organs detect the position of the head with respect to gravity and detect linear acceleration due to translational movements. These natural linear accelerometers, the utricle and the saccule, lie in roughly orthogonal planes. The utricle lies primarily in the horizontal plane and is sensitive to translational motion in the fore–aft and intra-aural directions. The saccule lies roughly in the vertical plane and is sensitive to motion in dorsal–ventral and fore–aft directions (Lindeman, 1969). The utricle is innervated by afferent fibers in the anterior division of the vestibular nerve with cell bodies in the superior vestibular ganglion, whereas most of the fibers innervating the saccule run in the posterior division of the vestibular nerve with cell bodies in the inferior vestibular ganglion (Gacek, 1969). Centrally, the linear acceleration signals are used in the creation of vestibular reflexes, including vestibulo-ocular and vestibulocollic responses. The appropriate oculomotor or cervicomotor response to a particular stimulation of the otolith organs is context dependent (Telford et al., 1997; Angelaki et al., 1999; Merfield et al., 1999). The central distribution of otolith afferents is important information to researchers seeking to understand processing of linear acceleration signals; including the interaction of these signals with other vestibular (semicircular canal) and other sensory information.
Whereas descriptions of central projections of the vestibular nerve date back to the late 19th century, the advent of specific neural tracers has advanced the ability to describe, in detail, the central anatomy of the vestibular nerve. Several tracers have been used, including tritiated amino acids (leucine and/or proline; Carleton and Carpenter, 1984; Schwarz and Schwarz, 1986), horseradish peroxidase (HRP; Siegborn and Grant, 1983; Kevetter and Perachio, 1986; Didier et al., 1987; Suarez et al., 1989; Burian et al., 1990; Cox and Peusner, 1990; Siegborn et al., 1991; Gstoettner et al., 1992; Lee et al., 1992; Naito et al., 1995; Dickman and Fang, 1996; Newlands et al., 2002), wheat germ agglutinin–conjugated horseradish peroxidase (WGA-HRP; Burian et al., 1990), horseradish peroxidase/cholera toxin mixture (Dickman and Fang, 1996), biocytin (Naito et al., 1995; Dickman and Fang, 1996), fluorescent carbocyanine dyes (Maklad and Fritzsch, 2002, 2003), and biotinylated dextran amine (BDA; Dino et al., 2001; Newlands et al., 2002). Study of specific vestibular end organs is only possible if a surgical technique can be devised that permits placement of the tracer in a specific area without damage or spread to adjacent end organs. A method for confirmation of the extent and accuracy of the injection site is also necessary. Tracing central projections from specific end organs has been accomplished in various species, including cats (Siegborn and Grant, 1983; Siegborn et al., 1991), guinea pigs (Didier et al., 1987; Burian et al., 1990; Gstoettner et al., 1992), chinchillas (Lee et al., 1992), gerbils (Kevetter and Perachio, 1986; Newlands et al., 2002), chickens (Cox and Peusner, 1990), squirrel monkeys (Naito et al., 1995), perinatal mice (Maklad and Fritzsch, 2002, 2003), and pigeons (Dickman and Fang, 1996).
Biotinylated dextran amine has become a popular molecule for anterograde neuroanatomic mapping (Brandt and Apkarian, 1992; Veenman et al., 1992). Either iontophoretic or pressure injections produce precise application of BDA to the target neuronal population without excessive leeching to adjacent areas. Transganglionic transport is seen, but transsynaptic labeling is not observed. In our previous studies, we have found this tracer to work well for the purposes discussed here (Newlands et al., 2002).
The existence of binocular foveate vision in primates requires complex visuovestibular interactions in guiding oculomotor responses during head motion induced by linear force. The interdependence between viewing distance, vergence angle, and the kinematic requirements of linear vestibular reflexes are different for primates than for lateral eyed animals (Schwarz et al., 1989; Paige and Tomko, 1991a, b; Schwarz and Miles, 1991; Telford et al., 1997). The major purpose of the current studies was to use a sensitive anterograde tracer technique to assess and compare central neuroanatomy of two otolith projections in macaques, with collection and analysis of vestibular end organs to ensure against the spread of the tracer to unintended end organs. These animals are widely used in studies of vestibular and oculomotor systems, because they demonstrate similar ocular motor range to humans and can be trained to perform the complicated behavioral tasks. Preliminary results have been presented (Vrabec et al., 1997a, b; Newlands et al., 2001).
Abbreviations
-
- BI
-
basal interstitial
-
- Cu
-
cuneate nucleus
-
- DCN
-
dorsal cochlear nucleus
-
- ECu
-
external cuneate nucleus
-
- Fl
-
flocculus
-
- GC
-
granular cell layer of the cerebellar cortex
-
- Gr
-
gracile nucleus
-
- I8
-
interstitial nucleus of the eighth nerve
-
- icp
-
inferior cerebellar peduncle
-
- IntA
-
interposed cerebellar nucleus, anterior part
-
- IntP
-
interposed cerebellar nucleus, posterior part
-
- L
-
cell group l
-
- Lat
-
lateral (dentate) cerebellar nucleus
-
- LVe
-
lateral vestibular nucleus
-
- Med
-
fastigial (medial cerebellar) nucleus
-
- mlf
-
medial longitudinal fasciculus
-
- MVeMC
-
medial vestibular nucleus, magnocellular part
-
- MVePC
-
medial vestibular nucleus, parvicellular part
-
- PC
-
Purkinje cell layer of the cerebellar cortex
-
- Pr
-
prepositus hypoglossi nucleus
-
- Pr5
-
principle sensory nucleus of the fifth cranial nerve
-
- scp
-
superior cerebellar peduncle
-
- Sol
-
solitary nucleus and tract complex
-
- sp5
-
spinal trigeminal tract
-
- Sp5
-
spinal nucleus of the fifth cranial nerve
-
- SpVe
-
spinal vestibular nucleus
-
- SuVe
-
superior vestibular nucleus
-
- VCA
-
ventral cochlear nucleus, anterior part
-
- VCP
-
ventral cochlear nucleus, posterior part
-
- VeCb
-
vestibulocerebellar nucleus
-
- VI
-
abducens nucleus
-
- VII
-
facial nucleus
-
- VIIn
-
facial nerve
-
- VIIIn
-
vestibulocochlear nerve
-
- VPFl
-
ventral paraflocculus
-
- XII
-
hypoglossal nucleus
MATERIALS AND METHODS
A total of 21 monkeys (18 Macaca fascicularis, 3 Macaca mulatta) were used in this study. All three rhesus monkeys (M. mulatta) had been used in single unit experiments and had electrode tracts in the vestibular nuclear complex. Before killing for histologic identification of recording sites, unilateral tracer injections were made into the utricular macula. None of the fascicularis monkeys had brainstem electrode tracts, although they were later used in acute studies on spinothalamic neurons conducted by other investigators. All procedures were performed according to the National Institutes of Health Guide for the Care and Use of Animals in Research and were reviewed and approved by the University of Texas Medical Branch's Institutional Animal Care and Use Committee (I.A.C.U.C. Protocol 90-05-054).
Injection of the otolith organs
These monkeys were anesthetized by using a combination of ketamine (5 mg/kg initial dose, supplemented as needed) and xylazine (10 mg/kg initial dose, supplemented as need) and postoperative pain was managed with buprenorphine hydrochloride (30 μg/kg). In seven animals (all M. fascicularis), injections were made into the saccular epithelium. The medial portion of the external canal was removed, allowing wide exposure of the middle ear and hypotympanum. The tympanic membrane, malleus, and incus were removed. The stapes was lifted out of the oval window, exposing the saccule.
In the other 14 animals (11 M. fascicularis, 3 M. mulata), the utricular neuroepithelium was injected. A transmastoid approach was used to expose the facial nerve. Again, the tympanic membrane and ossicles were removed to improve exposure. The nerve was lifted out of the fallopian canal from the cochleariform process to the stylomastoid foramen and displaced anteriorly. A vestibulotomy was created on the superior aspect of the stapes footplate to widely expose the ventral surface of the utricular macula.
A glass micropipette was filled with a solution of 5% BDA (Molecular Probes, 10,000 molecular weight) in 0.1 M phosphate buffer (pH 7.4) and its tip broken to a final diameter of 20 μm. The tip was guided into the saccule or utricle by using a micromanipulator. Gentle suctioning of the vestibule to remove the perilymph improved visualization of the pipette tip entering the neuroepithelium. BDA (200–250 nl) was pressure ejected from the tip into the neuroepithelium. At least two injections at different sites in the neuroepithelium were made in each animal. In two animals, 3,000 molecular weight BDA was used, but no central transport was seen.
Histologic processing
After a survival time of 14–35 days, the animals were injected with a lethal dose of anesthetic and perfused transcardially with warmed heparinized saline followed by a cold solution of fixative containing 4% paraformaldehyde and 0.1 M L-lysine in 0.1 M phosphate buffer (pH 7.4) and finally with 25% sucrose in the paraformaldehyde fixative. Next, the oval window was opened and the vestibule was directly bathed in fixative.
The brain was stereotaxically blocked, carefully extracted from the calvarium, and immersed in the 25% sucrose fixative solution for at least 1 week before cutting. The central tissue was cut in the transverse plane in 40 to 50 μm sections on a sliding microtome. The sections were incubated in 1:100 or 1:50 avidin–biotin (Vector Labs) and 0.25% Triton X-100 for 2.5 hours at room temperature or 18 to 48 hours at 5°C (Purcell and Perachio, 1997). A cobalt-intensified diaminobenzidine/glucose oxidase reaction (Kevetter and Perachio, 1986) demonstrated the BDA labeling. Sections were then rinsed again in phosphate buffered saline (pH 7.4), mounted on subbed slides, and cover-slipped. Sections were viewed with brightfield microscopy.
The temporal bones containing the exposed vestibular end organs were bathed in fixative for at least 24 hours after perfusion before the individual cristae and otolith organs were carefully extracted and placed in phosphate buffer (0.1 M, pH 7.4). The pigmented epithelium and membranous labyrinth of the crista ampullares and the otolithic membranes of the utricular and saccular macula were carefully teased from the sensory neuroepithelium to allow maximal visualization of the chromagen. The tissue was then immediately incubated in 1:100 avidin–biotin (Vector Labs) and 0.05% Triton-X 100 for 2.5 hours at room temperature (Purcell and Perachio, 1997). The tissue was then dehydrated through ethanol (50%, 70%, 95%, 100%) to 100% propylene oxide, and embedded in Epon resin. The end organs were trimmed and examined under light microscopy to verify the injection site and any spread to adjacent end organs.
Data analysis
All of the salvaged end organs were examined for spread of tracer. As with HRP (Kevetter and Perachio, 1986; Lee et al., 1992), spillage of BDA into the endolymphatic or perilymphatic space did not appear to result in transganglionic transport or labeling of ganglion cells. Therefore, injections were considered specific in the absence of spread of tracer to other end organs.
Every section of the central tissue was examined by several of the authors. No attempt was made to quantitatively evaluate the density of labeling at particular spots because labeling was so dependent upon the amount of tracer injected and subject to variability of the reaction intensity. For every experiment, qualitative estimates were made of the density of anterograde label were based on comparing the density of label in the different nuclei with the densest label that was observed in the area of heaviest labeling in that particular animal. The labeled fibers and terminals and the cytoarchitectonic borders of brainstem and cerebellar nuclei were represented using drawings of projected sections throughout the rostrocaudal extent of the labeled areas. All identification of anatomic structures is based on the rhesus monkey atlas of Paxinos et al. (2000). Cerebellar anatomy was further defined according to the atlas of Madigan and Carpenter (1971). Brightness, contrast, and sharpness were adjusted in captured images by using Adobe Photoshop software (version 6.0; Adobe Systems, Mountain View, CA) when deemed necessary (Saper, 1999).
RESULTS
End organ specificity
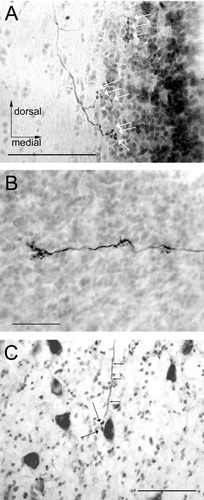
Examination of the end organs confirmed the restriction of the site of injection to the desired end organ in all but one case, which was discarded. All of the injections were subtotal and many were quite small. Analysis of end organs revealed dense staining at the injection site (Fig. 1). Utricular injections were purposefully concentrated in the posterior utricle to avoid damaging and labeling fibers from the anterior semicircular canal ampulla passing deep to the anterior utricle. Because the extent of the injection could not be precisely controlled, it was not feasible to perform systematic mapping of projections from difference quadrants of the macula.
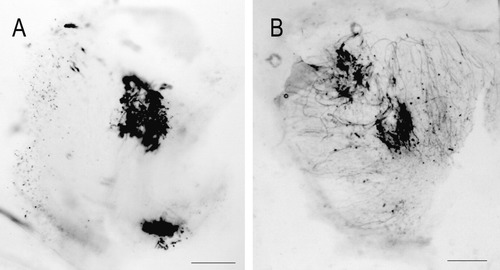
Two examples of end organ injections. A: Two injections into the utricular macula in fascicularis 285. B: Two injections into the saccular macula in fascicularis 284. These demonstrate clearly localized biotinylated dextran amine at the limited injection sites. Labeled afferent fibers are prominent in the surrounding neuroepithelium, particularly in B. Scale bars = 200 μm in A,B.
Saccular nerve
In four of the seven saccular injections, central labeling was attained and was the basis of our analysis. In the animals where no central transport was seen, we suspect that the injection was too superficial and did not disrupt the neuroepithelium enough to allow transport. The labeled fibers ran from the labyrinth in the posterior portion of the vestibular nerve. They branched at the lateral border of the lateral vestibular nucleus (LVe) into a descending branch that ran in the inferior and lateral portion of the spinal vestibular nucleus (SpVe) and a ventral branch that ran lateral to the LVe through and lateral to the superior vestibular nucleus (SuVe) to the cerebellum.
Afferent terminals were most prominent in the brainstem. Of the major vestibular nuclei, the SpVe was most thickly labeled (Fig. 2A). This nucleus was labeled throughout. The SuVe was most heavily labeled laterally (Fig. 2B). This region of the nucleus is parvicellular; the more central magnocellular region received little input. Some terminals were seen in the LVe (Fig. 2C), although this nucleus was very lightly labeled. The medial vestibular nucleus, magnocellular part (MVeMC) also received light projections, mostly on its lateral borders, with few terminals noted within the central regions of the nucleus. Almost no labeling was noted in the medial vestibular nucleus, parvicellular part (MVePC), except in the rostral portion of this nucleus where it borders the SuVe.
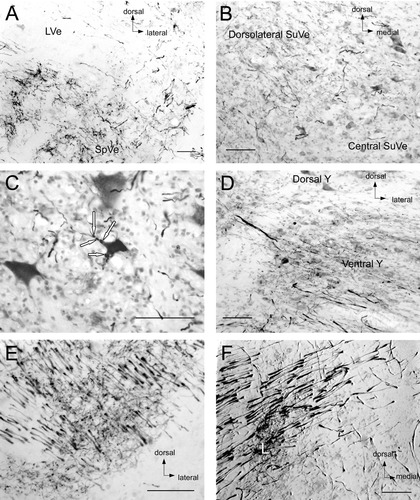
Central labeling after saccular macula injection in fascicularis 284. A: Rostral pole of the SpVe, demonstrating heavy terminal labeling and abundant descending fibers. The LVe dorsal to the SpVe shows a relative paucity of label. B: Labeling in the SuVe. The lateral portion of the nucleus is much more heavily labeled than the central portion. C: Terminals (white arrows) on a neuron in the LVe. D: Labeling in the ventral but not dorsal nucleus y. E: Interstitial nucleus of the eighth nerve terminals amongst saccular nerve fibers. F: Cell group l (L), lateral to the SpVe and LVe inferior to the entry of the vestibular nerve into the vestibular nuclei. All images oriented as in A. For abbreviations, see list. Scale bars = 100 μm in A–F.
The saccular nerve projected strongly to several accessory vestibular nuclei. Nucleus y was very heavily labeled (Fig. 2D). This labeling was essentially limited to the ventral subdivision of this nucleus. The interstitial nucleus of the eighth nerve (I8) was very densely labeled, particularly posterolaterally (Fig. 2E). Another very heavily labeled area (Fig. 2F) was just lateral to the SpVe, cell group l. Figure 3 demonstrates the distribution of labeling and fibers from representative coronal sections in one animal (fascicularis no. 284).
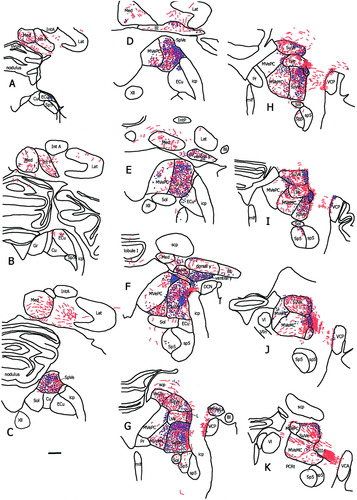
A schematic drawing of every eighth 50-μm coronal section through the vestibular nuclei in one animal (fascicularis 284) from caudal (A) to rostral (K). Red lines represent labeled fibers, and blue dots represent terminal fields. The density of the red lines and dots reflect the density of fibers and terminals present in that section. Anatomic boundaries are based on the atlas of Paxinos et al. (2000). For abbreviations, see list. Scale bar = 1 mm.
Heavy labeling was seen in the rostral, dorsal external cuneate nucleus (ECu; Fig. 4A). Labeling was also seen in the posterior part of the ventral cochlear nucleus (VCP). The VCP was labeled at its medial area, along the inferior cerebellar peduncle, and dorsally, along the floccular peduncle (Fig. 4B). The anterior part of the ventral cochlear nucleus was not labeled, except for a few terminals adjacent to the vestibular nerve root on the most posterior and medial aspect of this nucleus. Similarly, the dorsal cochlear nucleus was not labeled. Other brainstem areas with labeling included the spinal nucleus of V. The saccular nerve also projected lightly to the parvicellular reticular nucleus (Fig. 4C). Rare labeled fibers were observed crossing the midline at the level of the abducens nucleus, but terminals were not seen in the medial reticular formation or in the abducens or periabducens nuclei. It is probable that these fibers were retrogradely labeled efferent fibers. Several efferent cell bodies were identified bilaterally.
Saccular nerve labeling in the brainstem of fascicularis 284. A: External cuneate nucleus. Arrows point to the dorsal and lateral edge of the brainstem. B: En passant terminals in the VCP. C: Terminal field in the parvicellular reticular formation ventral to the rostral MVeMC. All orientation as in A. Scale bars = 100 μm in A–C.
Projections to the cerebellum passed through the juxtarestiform body and coursed adjacent to the fastigial (medial cerebellar) nucleus (Fig. 5A). Terminals were noted throughout the fastigial nucleus, although the density of labeling was much less than seen in the brainstem nuclei. The posterior division of the interposed cerebellar nucleus (Fig. 5B) and lateral (dentate) cerebellar nucleus displayed occasional terminal fields. Light labeling was also seen in vestibulocerebellar nucleus and the lateral extent of the basal interstitial cerebellar nucleus.
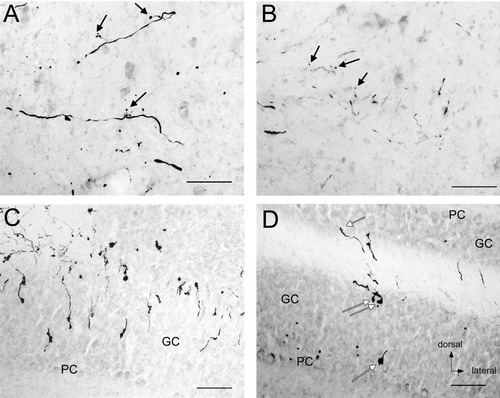
Saccular nerve labeling in the cerebellum of fascicularis 284. A: Terminals in fastigial nucleus. Arrows point to terminals. B: Terminals in the posterior interposed cerebellar nucleus. Arrows point to terminals. C: Heavy projections to the granular cell layer (GC) of vermal lobule IXd. PC, Purkinje cell layer. D: Multiple terminals (white arrows) in the granular cell layer in lobule VI. All images oriented as in D. Scale bars = 100 μm in A–D.
Cerebellar cortical projections were most prevalent in vermal lobule IX (uvula), particularly in division d (Fig. 5C). Vermal lobule X (nodulus) was also labeled, particularly toward the core of the lobule. Vermal lobules I, V, VI, and VIII also received afferent projections (Fig. 5D), although this projection was much weaker. Dense labeling was seen in the bottom of the sulcus between lobules IXd and X. All terminals were in the granular cell layer. No projections were found in more lateral areas, including the flocculus and ventral paraflocculus. Most terminals were ipsilateral to the injected saccule; however, a few vermal terminals were observed on the opposite side of the midline. A summary of the projections from the saccule is found in Table 1.
| Utricular | Saccular | |
|---|---|---|
| Vestibular nuclei | ||
| Spinal | +++ | ++++ |
| Superior | +++ | ++ |
| Medial (parvicellular) | ++ | + |
| Medial (magnocellular) | +++ | ++ |
| Lateral | 0 | + |
| Nucleus y | +++ | ++++ |
| Interstitial of eighth nerve | ++++ | ++++ |
| Group 1 | ++++ | +++ |
| Cerebellum | ||
| Vermal lobules I through VIII | +/0 | +/0 |
| Uvula | + | +++ |
| Nodulus | ++ | ++ |
| Flocculus | +/0 | 0 |
| Ventral paraflocculus | +/0 | 0 |
| Ipsilateral fastigial nucleus | + | + |
| Contralateral fastigial nucleus | + | 0 |
| Ipsilateral interposed posterior | +/0 | +/0 |
| Ipsilateral vestibulocerebellar | +/0 | +/0 |
| Ipsilateral basal interstitial | 0 | +/0 |
| Brainstem | ||
| External cuneate | + | ++ |
| Reticular nuclei | +/0 | + |
| Spinal nucleus of V | 0 | + |
| Ventral cochlear nucleus | 0 | + |
- 0, none; +/0, inconsistent or occasionally seen; +, light; ++, moderate; +++, heavy; and ++++, very heavy terminal labeling. Boldface type indicates projections that are heavier from one otolith organ than from the other.
Utricular nerve
In six of the 13 limited utricular injections central labeling was attained. Three of these animals demonstrated superior labeling and these two fascicularis and one rhesus are the primary source of results discussed here. Labeling in the remainder of the animals was less extensive but provided some confirmatory data. All of the reported projections were seen in at least two animals. The labeled fibers ran in the anterior portion of the vestibular nerve. They branched at the lateral border of the LVe, next to group l, into a descending branch that ran in the inferior and lateral portion of the SpVe and a ventral branch that ran lateral to the LVe through and lateral to the SuVe to the cerebellum.
The pattern of labeling after injection into the utricle differed significantly from the labeling described for saccular injections. Utricular nerve labeling was more widely distributed through the vestibular nuclei. The heaviest labeling was seen in the rostral part of the nuclei. In particular, the ventral aspect of the SuVe, the MVeMC, and the rostral pole of the MVePC were the most labeled areas (Fig. 6A–C). The SpVe had terminal labeling, especially rostrally, ventrally, and laterally. Utricular projections to nucleus y were also seen (Fig. 6C). These were also noted in the ventral subdivision of the nucleus, not the dorsal subdivision. Heavy labeling was seen at the bifurcation of the vestibular nerve in group l (Fig. 6D). Labeling seen in the interstitial nucleus of the eighth nerve was rostral to the area with labeling noted after saccular injection, reflecting the more rostral location of the utricular fibers. The areas of the interstitial nucleus of the eighth nerve labeled were predominantly lateral. Figure 7 demonstrates the distribution of terminal labeling and axonal fibers from representative coronal sections in one fascicularis (MA).
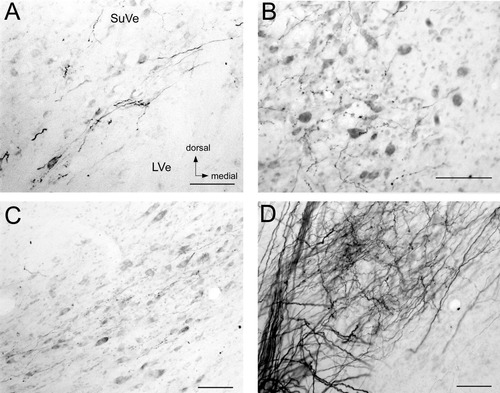
Utricular nerve labeling in the vestibular nuclear complex. A: Terminals and fibers in the SuVe. Terminals and fibers are seen along the ventral edge of this nucleus. B: Terminal labeling in the rostral MVePC. C: Terminals in nucleus y. A–C: All images from fascicularis MA. D: Fibers and terminals in cell group l at the bifurcation of the utricular nerve lateral to LVe and SpVe in rhesus CH. All images oriented as in A. For abbreviations, see list. Scale bars = 100 μm in A–D.
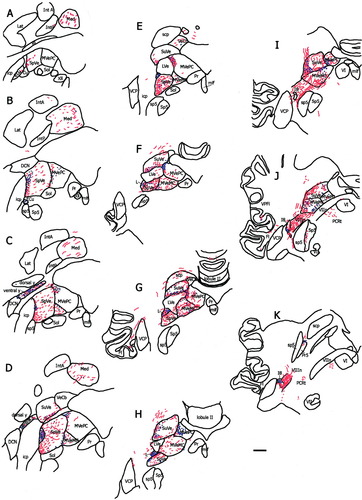
A schematic drawing of every eighth 50-μm-thick section through the vestibular nuclei from caudal (A) to rostral (K) in one fascicularis monkey (MA). Red lines represent labeled fibers, and blue dots represent terminals. The density of the red lines and dots reflect the density of fibers and terminals present in that section. This animal had light labeling, approximately 100 fibers, accounting for the less dense labeling compared with the larger saccular injection shown in Figure 3. For abbreviations, see list. Scale bar = 1 mm.
Reticular projections from the utricle were very light. Occasional terminals were seen in the parvicellular and gigantocellular reticular formation, although these findings were rare. The utricular nerve also projects directly to the ECu, although this input is weaker than that from the saccular nerve. Projections to the spinal nucleus of the trigeminal nerve were also present.
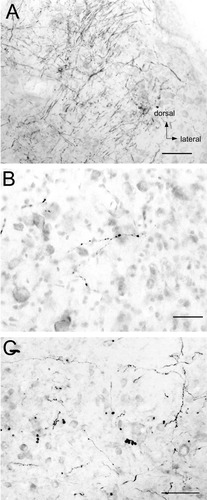
Cerebellar projections were distinctly less common. Projecting fibers passed mainly through the superior cerebellar peduncle. Sparse terminal fields were noted in the flocculus and ventral paraflocculus (Fig. 8A,B). Terminals were seen primarily in lobule X, although terminals in other lobules, notably II, V, and IX were seen. Cerebellar nuclear projections were seen in the fastigial nucleus, both ipsilateral and contralateral to the utricular injection (Fig. 8C). These fibers crossed the midline within the cerebellum as no contralateral brainstem projections were seen. A summary of the utricular projections is also presented in Table 1.
Utricular nerve labeling in the cerebellum of fascicularis MA. A: Terminals, highlighted by white arrows, demonstrating projections to the granule cell layer of the medial part of floccular folia 3. B: Ventral paraflocculus (floccular folia 6) terminals in the granular cell layer. C: Fiber (small arrows) and terminals (larger arrows) in the ipsilateral fastigial nucleus. All orientations as in A. Scale bars = 100 μm in A,C; 50 μm in B.
DISCUSSION
Projections to the major vestibular nuclei
For the purpose of comparison to the current investigation, we consider studies that directly addressed central terminations of the saccular and/or utricular nerve. The projections of these branches have been investigated in gerbils (Kevetter and Perachio, 1986; Newlands et al., 2002), pigeons (Dickman and Fang, 1996), cats (Siegborn and Grant, 1983; Imagawa et al., 1995, 1998—the latter two intracellular labeling studies), guinea pigs (Didier et al., 1987; Gstoettner et al., 1992), mice (Maklad and Fritzsch, 2002), and squirrel monkeys (Naito et al., 1995).
Superior vestibular nucleus.
The SuVe is involved as a relay nucleus in the vertical vestibulo-ocular reflex (VOR). Secondary VOR neurons project by means of the medial longitudinal fasciculus to the ipsilateral oculomotor and trochlear nuclei (Highstein, 1973; Highstein and Reisine, 1979; Graf et al., 1983; McCrea et al., 1987a). The nucleus receives extensively overlapping inputs from all three semicircular canals (Naito et al., 1995; Dickman and Fang, 1996; Newlands and Perachio, 2003).
In gerbils, saccular afferents had only sparse SuVe terminations (Kevetter and Perachio, 1986). In cats, saccular projections to the caudal SuVe are reported from two intracellularly filled units (Imagawa et al., 1998). Saccular projections to the SuVe are lateral in pigeons (Dickman and Fang, 1996), lateral in guinea pigs based on application of HRP crystals to the saccular nerve (Gstoettner et al., 1992) or injection into the saccular neuroepithelium (Didier et al., 1987), and ventrolateral in squirrel monkeys (Naito et al., 1995). In general, these findings are confirmed here in macaques but dorsal terminals are also noted in the rostral half of the nucleus.
Reports of utricular projection patterns in other species have varied. The utricle had terminations primarily in the lateral and rostral parvicellular portions of the nucleus in gerbils (Newlands et al., 2002). Siegborn and Grant (1983) did not find that utricular afferents projected to the SuVe in cats after HRP application to the cut nerve stump. Yet in squirrel monkeys, the utricular afferents were found to project to the dorsolateral SuVe (Naito et al., 1995), whereas single unit fills of four neurons in cats demonstrated projection to the caudal and ventral portion of the nucleus only (Imagawa et al., 1995). The current study demonstrates a utricular projection that is primarily to the ventral and lateral parts of the nucleus.
All of these reports demonstrate that both otolith macula project to peripheral regions of the SuVe, not to the central, larger-celled area thought to be the origin of inhibitory second-order vestibulo-ocular fibers (Highstein and Reisine, 1979; Mitsacos et al., 1983a). These peripheral regions have been shown to project to the cerebellum and bilateral vestibular nuclei (Gacek, 1978; Pompeiano et al., 1978; Mitsacos et al., 1983b).
Lateral vestibular nucleus.
The LVe is characterized by Deiters' cells, which project to and receive inputs from the spinal cord (Wilson et al., 1967; Boyle and Pompeiano, 1980; Boyle et al., 1992; Xiong and Matsushita, 2001) and, thus, is primarily believed involved in vestibulospinal mechanisms. Neurons in the lateral and central LVe give rise to the lateral vestibulospinal tract and participate in postural control (Wilson et al., 1967; Boyle et al., 1992). Neurons in the lateral vestibulospinal tract project to the cervical and lumbar spinal cord. The lumbar-projecting neurons bypass the cervical cord and do not contribute to head posture and stabilization (Boyle, 2000). These neurons are located more dorsally and rostrally in the LVe of cats, where utricular and saccular afferent terminals are not seen, consistent with monosynaptic inputs being more prevalent to cervical projection than to lumbar-projecting LVe cells. Lateral vestibulospinal tract neurons projecting to the cervical cord arise from both the LVe and MVeMC, and many of these show physiologic evidence of monosynaptic afferent input (Boyle et al., 1992; Uchino et al., 1994; Sato et al., 1996). Although the LVe has been considered void of afferent input, more recent studies using BDA (Kevetter et al., personal communication; gerbil) and carbocyanine fluorescent dyes (Maklad and Fritzsch, 2002; mouse) have reported afferent terminals in the LVe. Similarly, in this study using BDA, such terminals are reported, although this connection is weak. Monosynaptic latencies were seen for production of excitatory postsynaptic potentials (EPSPs) in many spinal-projecting LVe neurons in squirrel monkeys (Boyle et al., 1992). These EPSPs were small and did not result in discharge, suggesting that afferent input to spinal-projecting LVe neurons are distal on the dendritic tree and may be outside the anatomic boundaries of the LVe (Highstein et al., 1987; Boyle et al., 1992).
Medial vestibular nucleus, parvicellular part.
This nucleus has medium to small neurons and is believed to be involved in projection to oculomotor nuclei, the medial vestibulospinal tract, and the vestibular commissural system (Gacek, 1969; Newlands et al., 1989; Minor et al., 1990; Boyle et al., 1992; Xiong and Matsushita, 2001). This nucleus receives input from all of the semicircular canals (Kevetter and Perachio, 1985, 1986; Sato et al., 1989; Siegborn et al., 1991; Gstoettner et al., 1992; Naito et al., 1995; Dickman and Fang, 1996). In general, the rostral portion of the nucleus is believed more involved in ocular motor reflexes and the caudal portion with spinal projections, although there is great overlap in these systems and many neurons project in both systems (Graf and Ezure, 1986; Highstein et al., 1987; McCrea et al., 1987a, b; Boyle et al., 1992; Büttner-Ennever, 1992; Scudder and Fuchs, 1992).
In contrast to canal inputs, otolith inputs do not ubiquitously occupy the MVePC. In this study, we found saccular projections were light, except for the lateral dorsal corner of the nucleus where it abuts the SuVe and LVe, which is consistent with the preponderance of previous reports. Saccular projections to the MVePC were not seen in gerbils (Kevetter and Perachio, 1986) and were reported as weak in cats based on single unit injections (Imagawa et al., 1998). Didier et al. (1987) found saccular projections to the rostral extent of the MVePC in guinea pigs. Naito et al. (1995), in squirrel monkeys, reported light saccular nerve projections throughout the MVePC. Potentially, in this latter study, there may have been spread of tracer to adjacent end organs or these findings may represent a species difference.
Utricular nerve afferents in gerbils project to the entire MVePC, except for the most central portion of the nucleus (Newlands et al., 2002). The utricular nerve was noted to project to the MVePC in cats (Siegborn and Grant, 1983, specifics not given) and squirrel monkeys (Naito et al., 1995, central part of the nucleus rostrally becoming more lateral as one moves caudally). After injection into single utricular units, Imagawa et al. (1995) described the distribution of terminals as similar to canals but sparser. In pigeons, the utricular and saccular projection was described as lateral (Dickman and Fang, 1996). The current study demonstrates limitation of utricular nerve terminals to the lateral border of the nucleus where it abuts the SpVe and the MVeMC and the rostral pole of the nucleus where it abuts the SuVe. In these locations, the afferents might be involved in ocular motor or spinal reflexes (Boyle et al., 1992). This region contains convergent central neurons that are sensitive to linear and angular acceleration but do not have eye movement sensitivity (Dickman and Angelaki, 2002) and, thus, are potentially involved in the medial vestibulospinal tract. Similarly, neurons believed involved in the VOR with both eye movement and vestibular sensitivity such as position vestibular pause or eye head velocity neurons are located in this region (Scudder and Fuchs, 1992). Many of these cell types have both angular and linear sensitivity and are potentially involved in control of both the linear and rotational VOR (Angelaki et al., 2001).
Medial vestibular nucleus, magnocellular part.
Older studies reporting afferent input to the ventral and ventrorostral LVe likely refer to the MVeMC. Studies tracing the individual canal (Siegborn and Grant, 1983; Kevetter and Perachio, 1986; Siegborn et al., 1991; Gstoettner et al., 1992; Naito et al., 1995; Kevetter et al., personal communication) and otolith nerves (Siegborn and Grant, 1983; Kevetter and Perachio, 1986; Didier et al., 1987; Naito et al., 1995; Newlands et al., 2002) have found that all of the end organs project significantly to this region.
In macaques, this nucleus receives both saccular and utricular input, although the utricular input is more substantial. The MVeMC is at the center of major output pathways of the vestibulomotor activity. Neurons mediating both the horizontal (McCrea et al., 1987b) and vertical (Graf and Ezure, 1986; McCrea et al., 1987a) VOR and vestibulospinal neurons are found in this area (Boyle and Pompeiano, 1980; Boyle et al., 1992). Lateral vestibular nucleus neurons described as driven monosynaptically from the saccular nerve potentially actually reside in the MVeMC, as reports are imprecise about the anatomic location of these cells (Uchino et al., 1997; Sato et al., 2000). The projection of all of the end organ afferents to the MVeMC provides an opportunity for interaction between utricular and canal inputs on central neurons.
The ascending tract of Deiters originates in the MVeMC and rostral MVePC and projects to the ipsilateral oculomotor nucleus subdivision that drives the medial rectus muscle (Baker and Highstein, 1978; McCrea et al., 1987b). Neurons in this tract carry combined angular and linear VOR signals that are likely involved in disconjugate eye movements, because their gain changes with viewing distance (Chen-Huang and McCrea, 1998). Many of these neurons behave like eye head velocity cells, which are known to carry both rotational and translational sensory information (Angelaki et al., 2001) and may be floccular target neurons (Lisberger et al., 1994). Other reports have noted type I pause vestibular pause neurons in the ascending tract of Deiters (McCrea et al., 1987a). The MVeMC also has many neurons projecting to the contralateral abducens (McCrea et al., 1987a). Thus, utricular inputs to this region are likely used to generate the linear VOR.
Spinal vestibular nucleus.
This nucleus projects to the spinal cord, the contralateral vestibular nuclei, and the vermis (Boyle and Pompeiano, 1980; Carleton and Carpenter, 1983, Newlands et al., 1989; Thunnissen et al., 1989; Boyle, 1993; Xiong and Matsushita, 2001). The rostral portion of the nucleus also participates in vestibulo-ocular pathways (Graf and Ezure, 1986). It receives input from the contralateral fastigial nucleus (Sugita and Noda, 1991), ipsilateral vermis (Carleton and Carpenter, 1983; Thunnissen et al., 1989), contralateral vestibular nuclei (Carleton and Carpenter, 1984; Newlands et al., 1989), and the spinal cord (Rubertone and Haines, 1982). Uchino et al. (1997) found saccular driven cells in the SpVe, some with monosynaptic input from one side of the striola and disynaptic convergence from the other side of the striola.
In macaques, the SpVe is most heavily innervated by saccular afferents. These afferents are found throughout the SpVe. Utricular projections to this nucleus are found rostrally, ventrally, and laterally. Several authors have described similar specific peripheral innervation patterns. In gerbils (Newlands et al., 2002), pigeons (Dickman and Fang, 1996), squirrel monkeys (Naito et al., 1995), and cats (Imagawa et al., 1995), utricular afferents were found primarily to project to the lateral portion of the nucleus. A strong saccular input was seen throughout the nucleus in these same species (Kevetter and Perachio, 1986; Naito et al., 1995; Dickman and Fang, 1996; Imagawa et al., 1998). Many lateral vestibular spinal tract neurons to the lumbar cord arise in the caudal SpVe (Boyle et al., 1992). The heavy saccular projection throughout this nucleus supports the role of the saccule in vestibulospinal righting reflexes.
Projections to accessory vestibular nuclei
Nucleus y.
In many mammals, the ventral portion of nucleus y receives saccular input (Gacek, 1969; Fredrickson and Trune, 1986; Kevetter and Perachio, 1986; Didier et al., 1987; Gstoettner et al., 1992; Naito et al., 1995), whereas the dorsal portion does not. The ventral portion, not the dorsal portion, participates in the vestibular commissural system (Gacek, 1978) and projects to the ipsilateral vestibular nuclei (Rubertone et al., 1983). In contrast, the dorsal portion of nucleus y receives the densest floccular outflow (Langer et al., 1985a) and projects to the oculomotor (Gacek, 1977; Highstein and Reisine, 1979; Steiger and Büttner-Ennever, 1979) and trochlear (Gacek, 1979) nuclei by means of the brachium conjunctivum. In rhesus macaques, Langer et al. (1985b) refer to the ventral portion of nucleus y as the supravestibular nucleus and demonstrate afferent projection to the flocculus (ipsilateral greater than contralateral). Ipsilateral but not contralateral nucleus y projections to the flocculus and bilateral nucleus y projections to the uvula were reported in galagos (Rubertone and Haines, 1981). The physiologic role of ventral nucleus y is not clear. Presumably, this nucleus could influence postural and ocular motor reflexes through extensive connections in the vestibulocerebellum and other vestibular nuclei.
There have been few reports of other vestibular nerve afferent projections to nucleus y. In squirrel monkeys, no utricular or canal projections were seen to nucleus y (Naito et al., 1995). In cats, one of the 16 intracellularly labeled regularly firing lateral canal units terminated in nucleus y; none of the six irregular fibers were traced to this nucleus (Sato et al., 1989). In gerbils, injections into canal ampullae did not result in labeling in nucleus y (Kevetter and Perachio, 1985, 1986; Kevetter et al., personal communication) but utricular afferents to nucleus y and the infracerebellar nucleus have been reported (Newlands et al., 2002). Didier et al. (1987), working in guinea pigs, found that only the posterior branch of the saccular nerve projected to the nucleus y group, whereas fibers traveling with the anterior branch did not. In the current study, both the saccular and utricular afferents projected to ventral nucleus y. As in our previous study in gerbils, dorsal nucleus y did have an occasional utricular terminal (Newlands et al., 2002).
Interstitial nucleus of the eighth nerve.
Little is known about this nucleus, other than its input from vestibular afferents. Input from the cerebellar cortex of the vermis has been reported in galagos (Haines, 1977) and the nucleus projects to the flocculus (Rubertone and Haines, 1981; Langer et al., 1985b) and nodulus (Rubertone and Haines, 1981) in primates.
Neuronal tracer studies have demonstrated, although inconsistently, input from all of the vestibular end organs. HRP tracer studies of the canal and otolith nerves in squirrel monkeys demonstrated little input from canals that was generally rostral and a heavier input from the utricle caudocentrally and saccule caudally (Naito et al., 1995). Similarly, we saw primarily lateral interstitial nucleus of the eighth nerve labeling in macaques. Intracellular studies in cats have demonstrated branches from afferents of all canals (Mannen et al., 1982; Sato et al., 1989) and the saccule (Imagawa et al., 1998). In gerbils, input arises from all end organs (Kevetter and Perachio, 1986; Newlands et al., 2002; Kevetter et al., personal communication).
Cell group l.
Group l is a small group of cells just lateral to the LVe that has been distinguished in macaques (Brodal, 1984), cats (Brodal and Pompeiano, 1957), galagos (Rubertone and Haines, 1982), and squirrel monkeys (Naito et al., 1995). Little is known about the connections of this area, but in cats, the cells appear to project to the spinal cord (Pompeiano and Brodal, 1957). For this reason, this cell group has been thought of as being functionally a part of the LVe (Pompeiano and Brodal, 1957). In squirrel monkeys, terminals from utricular and saccular afferents were seen in cell group l (Naito et al., 1995). In the current study, we saw a group of smaller cells beginning between the rostral pole of the SpVe and the inferior cerebellar peduncle extending up to the entry zone of the eighth nerve. This group of cells is ventral to nucleus y and ventral and caudal to the lateral, caudal pole of SuVe. These cells are heavily labeled after injection of either the utricular or saccular macula, although the utricular input appeared stronger.
Projections to other brainstem nuclei
Cochlear nucleus.
Several studies have described saccular innervation of the cochlear nuclei. Kevetter and Perachio (1985, 1986, 1989) described this projection in gerbils. Single unit labeling and injection of HRP into the macula demonstrated terminations in the anterior and posterior portions of the ventral cochlear nucleus and the dorsal cochlear nucleus. Most of these terminations were in the granule cell domain, especially in the subpeduncular corner between the anteroventral cochlear nucleus and the floccular peduncle of the cerebellum. Burian and Gstoettner (1988) also showed saccular afferents terminating between the dorsal and posteroventral cochlear nuclei. Gstoettner et al. (1992), also in guinea pigs, demonstrated saccular projections to the dorsal cochlear nucleus. In the current study, we see clear evidence of a saccular projection in rhesus monkeys to the VCP that is strongest at the medial edge of this nucleus abutting the inferior cerebellar peduncle. We did not, however, examine the cochlea for spread. Given the lack of label in the cochlear nerve and other parts of the cochlear nuclei, contamination seems unlikely. Acoustically responsive saccular nerve fibers have been demonstrated in cats, although the role of such fibers and of the saccular afferent projection to the cochlear nucleus remains obscure (McCue and Guinan, 1994).
External cuneate nucleus.
Projections to the superior part of the ECu have been irregularly described originating from several end organs. In gerbils, this projection has been described from the utricle (Newlands et al., 2002) and saccule (Kevetter and Perachio, 1985, 1986). In cats, Siegborn and Grant (1983) reported this projection from the lateral canal but not the utricle. In guinea pigs, a saccule to ECu projection has also been reported (Didier et al., 1987). We find clear projections to this nucleus from the saccule and utricle in macaques. The ECu carries proprioceptive input from the neck and forelimb to the thalamus and cerebellum in monkeys (Rustioni et al., 1979; Pearson and Garfunkel, 1983). Thus, this nucleus may be a center for integration of proprioceptive and otolith inputs that relay a signal related to the animal's position space to higher levels in the brain (Jensen and Thompson, 1983; Anastasopoulos et al., 1991).
Projections to the cerebellum
Cerebellar nuclei.
Based on degeneration, Carpenter (1960) described cerebellar-projecting vestibular afferent fibers to the fastigial nucleus of cats, whereas Brodal and Høivik (1964) and Korte and Mugnaini (1979) saw fibers passing through but were not able to determine whether they were fibers of passage or terminals. In rats, Mehler and Rubertone (1985), by using degeneration techniques, noted fibers to the caudal fastigial nucleus only. HRP studies in gerbils have demonstrated saccular projections to the lateral and interposed cerebellar nuclei (Kevetter and Perachio, 1986) and utricular projections to the fastigial and anterior interposed cerebellar nuclei (Newlands et al., 2002). Lateral and anterior canal afferents terminate in the interposed nucleus as well (Kevetter et al., personal communication). All of these projections described in gerbils are on a minute scale.
In the current study, projections to the fastigial nuclei were seen bilaterally from the utricle and unilaterally from the saccule. Although a stray terminal from the saccule was seen in other cerebellar nuclei, these terminals are rare. Our study agrees with others that vestibular afferent input to the cerebellar nuclei is weak, and primarily involves the fastigial nucleus. Thus, complex otolith responses recorded in the fastigial nucleus are likely manifestations of polysynaptic inputs (Zhou et al., 2001).
Vermis.
Many studies have demonstrated a substantial vestibular afferent input to the vermis, but few have been designed to distinguish the otolith contribution. Injection of tritiated amino acids into the labyrinth demonstrated heavy labeling of mossy fiber rosettes in the granular layer of the ipsilateral uvula and nodulus and lighter labeling in the deep aspects of vermal folia I, V, and VI (Carleton and Carpenter, 1984). Similar findings were reported in rabbits (Gerrits et al., 1989) where heavy labeling was noted in the ipsilateral and medial third of the contralateral vermal lobules X and IXd after injection of WGA-HRP and tritiated leucine in the vestibular ganglion. Weak labeling was seen in lobules I, II, and the fissures between lobules IV and V and V and VI.
These anterograde studies have been complemented by retrograde studies as well. Injection of HRP into the nodulus of five rabbits demonstrated Scarpa's ganglion labeling, whereas injections into lobule VI did not (Alley et al., 1975). Large HRP injections into the vermis in cats also retrogradely labeled Scarpa's ganglion cells bilaterally (Kotchabhakdi and Walberg, 1978). Injection of WGA-HRP into lobules IXd and X retrogradely filled 64% of Scarpa's ganglion cells in rabbits (Barmack et al., 1993). The authors considered 64% a conservative estimate of the strength of the vestibular afferent projection to the vermis. They confirmed the projection by injection of C fragment of tetanus toxin into the labyrinth and observing dense labeling ipsilaterally in vermal areas. Weak labeling was seen in the primary fissure and between the third and fourth lobules. They also injected fluorescent markers into the uvula–nodulus and vestibular nuclear complex and found double fluorescent labeling in more than 50% of Scarpa's ganglion neurons.
Retrograde and anterograde tracer studies in gerbils have confirmed projections from all end organs to the vermis. The posterior canal projected to the uvula and nodulus, whereas the saccule projected to lobules I, V, VI, and, most strongly, the uvula (Kevetter and Perachio, 1986). Utricular, anterior canal, and lateral canal afferents project most heavily to the uvula and nodulus (Kevetter et al., personal communication; Newlands et al., 2002). The afferents in this species make up mossy fibers that terminate on the granule cells and unipolar brush cells (Dino et al., 2001). Injection of BDA into the uvula and nodulus demonstrated that the majority of utricular and saccular afferents to the vermis were dimorphic and arose from the peristriolar zone (Purcell and Perachio, 2001). Didier et al. (1987) noted that projections to the nodulus travel through the posterior branch but not the anterior branch of the saccular nerve in guinea pigs. In perinatal mice, saccular and utricular afferents project primarily to the uvula, subfolium IXd, with little projection to the nodulus, whereas canal afferents project primarily to the nodulus, with less input to IXd (Maklad and Fritzsch, 2003).
The uvula and nodulus have a rich convergent input of vestibular and visual signals (Barmack and Shojaku, 1995). Climbing fibers from the β-nucleus and dorsomedial cell column of the inferior olive carry vestibular inputs related to coding dynamic roll information in the plane of the vertical canals and static tilt information, presumably of utricular origin (Barmack and Shojaku, 1995). Mossy fiber input to the uvula/nodulus arises from the SuVe, rostral and caudal MVePC, and SpVe, whereas the LVe does not project to this area and the MVeMC projections are light in rabbits (Thunnissen et al., 1989; Barmack et al., 1992). These connections were found to be reciprocal and some vestibular nucleus neurons that project to the uvula/nodulus also project to the flocculus (Epema et al., 1990). The convergence of primary and secondary vestibular information in the uvula/nodulus makes this area an ideal candidate for control and modification of spatial orientation tasks. Evidence for the uvula/nodulus in this role is the finding that lesions of the uvula and nodulus in rhesus monkeys eliminate the ability of the otolith system to generate steady-state nystagmus during constant velocity rotation and prevent otolith contribution to the VOR during low-frequency sinusoidal off-vertical axis rotations (Angelaki and Hess, 1995).
Surprisingly, we found light input to lobule IX from the utricular injections. This contrasts with a very strong projection to lobule IXd with a weaker input to lobule X seen after saccular injection. This finding suggests a difference in utricular and saccular nerve projections, although the subtotal nature of our injections raises the possibility that our finding reflects a subtotal sampling of the otolith to vermis projection.
Flocculus and paraflocculus.
The presence or absence of afferent projections to the flocculus and paraflocculus is controversial. Degeneration studies suggest such a projection but are inconclusive (Korte and Mugnaini, 1979). Tritiated amino acid injections into the labyrinth of cats demonstrate limited labeling in the flocculus but no labeling in the paraflocculus (Carleton and Carpenter, 1984). Electrophysiologic studies of this region have not provided definitive answers, but in rabbits (Ito et al., 1982) and cats (Shinoda and Yoshida, 1975), data have been obtained to suggest a direct afferent input to the flocculus.
Several retrograde and anterograde tracer studies have examined vestibular afferent projections to the flocculus and paraflocculus. A small number of ipsilateral vestibular ganglion cells were labeled after HRP injection into the flocculus in rats (Blanks et al., 1983). In rabbits, Gerrits et al. (1989) injected WGA-HRP and tritiated leucine into the vestibular ganglion. None of the labeled afferents projected to the flocculus or paraflocculus. These authors believe that earlier retrograde studies in rabbits (Alley et al., 1975) and rats (Blanks et al., 1983) with injection into the flocculus involved spread of the tracer to nucleus y, giving a false-positive result. Also in rabbits, injection of WGA-HRP and fluorescent dyes into the flocculus and C fragment of tetanus toxin into the vestibular ganglion demonstrated essentially no vestibular afferent projection to the ventral paraflocculus in rabbits and a weak projection to the flocculus (Barmack et al., 1993).
Osanai et al. (1999) injected HRP, WGA-HRP, Fast Blue, or diamidino yellow into the flocculus, ventral paraflocculus, and/or dorsal paraflocculus of rats. After flocculus injection, these authors reported vestibular ganglion labeling in several but not all of their experimental animals. In the limited injections to the flocculus, no retrograde vestibular ganglion labeling was seen, whereas in the large injections labeling was seen, possibly from spill into nucleus y. Injection into the dorsal or ventral paraflocculus did not produce labeling in the vestibular ganglion. BDA injection into the flocculus of gerbils labeled peripheral zone dimorphic units in all of the semicircular canals but not afferents originating in the otolith organs (Purcell and Perachio, 2001).
As with these afoveate animals, results of anatomic studies in monkeys have yielded inconsistent results. Langer et al. (1985b) injected HRP into the flocculus of rhesus monkeys and reported no retrograde vestibular afferent labeling. In Macaca fuscata, WGA-HRP injection into the flocculus resulted in labeling in 5–10% of the vestibular ganglion cells, whereas injections into the ventral paraflocculus did not retrogradely label vestibular ganglion neurons (Nagao et al., 1997). These authors contend that only those folia caudal to the posterolateral fissure in monkeys (folia 1–5; after Madigan and Carpenter, 1971) are comparable to the flocculus in other species, including afoveate animals. The rest of the folia (6 through 10) are the ventral paraflocculus. These folia include the area suggested by Miles and Lisberger (1981) to be involved in motor learning in the VOR. They found that injection into the ventral paraflocculus (rostral to posterolateral fissure) did not demonstrate input from the vestibular ganglion. None of the retrograde studies in monkeys include histologic examination of the vestibular neuroepithelium to address which end organ is innervated by vestibular afferents that project to the flocculus or ventral paraflocculus.
We demonstrate evidence of clear, albeit weak, utricular projection to the flocculus and ventral paraflocculus. The role of this region in VOR gain adaptation suggests an interesting potential role for these afferents, particularly in the mediation of the VOR gain appropriate for vergence during the translational VOR. However, the significantly greater inputs to the cerebellar cortex from the MVeMC, MVePC, interstitial nucleus of the eighth nerve, and nucleus y suggest that the disynaptic pathway is more dominant for conveying translational motion information to the flocculus and ventral paraflocculus. The convergence of these two sources of utricular-related inputs to the flocculus regions with visual-mediated signals relayed from the nucleus prepositus hypoglossi and the dorsal cap of the inferior olive (Langer et al., 1985b; Takeda and Maekawa, 1989) could underlie the mechanisms for visual modification of the translational VOR.
Summary
In this study, we demonstrated the central projections of the macular nerves in macaques. In the major vestibular nuclei, there was a greater input to the superior and medial nuclei from the utricle and more input to the spinal nucleus from the saccule. This finding is suggestive of a more prominent role for the utricule in vestibulo-ocular functions because of the strong presence of neurons in the more rostral vestibular nuclei whose responses have been implicated in those functions. Clearly, this is not the sole macular influence on the VOR, because saccular projections do involve those areas, VOR-projecting neurons are found in more caudal areas, and many vestibular neurons appear involved in both ocular motor and spinal projection pathways. The prominence of saccular input to the spinal nucleus and the lateral vestibular nucleus is consistent with its presumed mediation of vestibulospinal functions. Moreover, the presence of utricular terminals in those and other areas that contain spinal-projecting neurons is supportive of a role for both otolith organs in postural control and equilibrium. In total, the significant overlap of utricular and saccular afferents suggest convergence of these inputs appropriate for functional integration for vestibular reflexes that depend on information that encompass detection of linear forces in a three-dimensional coordinate framework. Projection of both macular nerves to the uvula and nodulus are consistent with a role for these structures in spatial orientation, whereas utricular afferent input to the flocculus suggests a role for this structure in translational VOR responses.
Acknowledgements
We thank Dr. William Willis for use of his fascicular macaques and Golda Anne Kevetter for helpful input. We also thank Melody Kaliff and Heather McMullen for technical assistance and Bonnie Regini for secretarial assistance.




