Chemically and morphologically identifiable glomeruli in the rat olfactory bulb
Abstract
Primary olfactory neurons that express the same odorant receptor are distributed mosaically throughout the olfactory neuroepithelium lining the nasal cavity, yet their axons converge and form discrete glomeruli in the olfactory bulb. We previously proposed that cell surface carbohydrates mediate the sorting out and selective fasciculation of primary olfactory axons en route to glomeruli. If this were the case, then axons that terminate in the same glomerulus would express the same complement of cell surface carbohydrates. In this study, we examined the expression of a novel carbohydrate (NOC-3) on neural cell adhesion molecule in the adult rat olfactory system. NOC-3 was expressed by a subset of neurons distributed throughout the olfactory neuroepithelium. The axons of these neurons entered the nerve fiber layer and terminated in a subset of glomeruli. It is interesting to note that we identified three unusually large glomeruli in the lateral, ventrolateral, and ventromedial olfactory bulb that were innervated by axons expressing NOC-3. NOC-3-expressing axons sorted out and fasciculated into discrete fascicles prior to entering these glomeruli. Each of these glomeruli was in a topographically fixed position in the olfactory bulbs of the same animal as well as in different animals, and their lengths were approximately 10% of the total length of the bulb. They could be identified reliably by both their topographical position and their unique morphology. These results reveal that axons expressing the same cell surface carbohydrates consistently target the same topographically fixed glomeruli, which supports a role for these molecules in axon navigation in the primary olfactory nerve pathway. J. Comp. Neurol. 436:497–507, 2001. © 2001 Wiley-Liss, Inc.
Primary olfactory neurons express one of ≈1,000 odorant receptors and are distributed mosaically throughout one of four semiannular zones in the nasal cavity (for review, see Mombaerts, 1999). The axons of these neurons initially are intermingled with axons of neurons that express different odorant receptors; however, when they reach the olfactory bulb, they sort out and target specific glomeruli. Neurons that express the same odorant receptor typically project their axons to topographically fixed glomeruli in the ipsilateral olfactory bulb (Vassar et al., 1993, 1994; Ressler et al., 1994; Mombaerts et al., 1996; Royal and Key, 1999).
Relatively little is known about the molecular mechanisms underlying the sorting out and targeting of axons in the olfactory nerve pathway. Molecules like neural cell adhesion molecule (N-CAM), L1, and TAG-1 are expressed widely and probably are involved in axon fasciculation (Whitesides and La Mantia, 1995; Treloar et al., 1997). Olfactory cell adhesion molecule (O-CAM), which is expressed in several zones of the olfactory neuroepithelium (Yoshihara et al., 1997), may contribute to partitioning axons into large, spatially defined bundles (e.g., ventrolateral vs. dorsomedial). However, to enable axons from neurons expressing the same odorant receptor to target specific, topographically fixed glomeruli, more specific guidance mechanisms must exist. Whereas the odorant receptors themselves appear to be involved in guiding olfactory axons (Mombaerts et al., 1996), particularly in the final stages of targeting, other cues are needed for axon sorting and fasciculation in the nerve fiber layer.
Carbohydrates often are expressed on cell surface molecules, such as N-CAM, and are expressed widely in the nervous system. Although N-CAM is expressed by all primary olfactory neurons (Gong and Shipley, 1996), some discrete isoforms of N-CAM express specific carbohydrates. NOC-3 and NOC-4 are present only on the 180-kD and 240-kD glycoforms of N-CAM, respectively, and these glycoforms of N-CAM are expressed by subpopulations of primary olfactory neurons. Galactose N-Cam (Gal-N-CAM), a discrete glycoform of N-CAM that has a terminal α-galactose (Mr, 205 kD), also is expressed by a restricted subpopulation of primary olfactory neurons (Pays and Schwarting, 2000). In contrast, a 205-kD fucosylated form of N-CAM is expressed by all primary olfactory neurons (Pestean et al., 1995). Thus, N-CAM has various glycosylated forms that are either expressed ubiquitously or are present on restricted subpopulations of primary olfactory neurons. This restricted expression of N-CAM glycoforms may provide a mechanism for selective fasciculation of axons.
Lectin histochemistry and antibody immunohistochemistry studies have found that many other carbohydrates in addition to the N-CAM glycoforms are present on subsets of primary olfactory neurons (see, e.g., Mori et al., 1985; Schwarting and Crandall, 1991; Key and Akeson, 1993; Saito et al., 1999). Combinations of the vast array of different carbohydrates may provide a coding mechanism that contributes to the fasciculation of discrete subsets of primary olfactory axons in the nerve fiber layer through interactions with carbohydrate-binding proteins, such as galectin-1 (Puche et al., 1996; St. John and Key, 1999). Neurons that express the same odorant receptor and target a specific glomerulus also may express a unique combination of cell surface carbohydrates.
To begin to address this question, we have analyzed the convergence pattern of a subpopulation of neurons that express NOC-3, a novel glycoform of N-CAM (Dowsing et al., 1997). We show here that NOC-3 axons target at least three topographically fixed glomeruli in the ventrocaudal olfactory bulb of adult rat. Because olfactory axons that express the same odorant receptor target individual glomeruli, our results provide two important insights regarding chemical coding in the olfactory system: First, specific carbohydrates are expressed by neurons that express different odorant receptors, and, second, axons that express the same odorant receptor can express the same cell surface carbohydrate. Cell surface carbohydrates, therefore, are not expressed randomly by different subpopulations of primary olfactory neurons. Instead, their spatial expression pattern is regulated tightly and is consistent with the specificity observed for odorant receptors, albeit they are expressed in larger subpopulations. We propose that cell surface carbohydrates provide chemical heterogeneity to the population of olfactory axons that contributes to the targeting of axons to individual glomeruli in the olfactory bulb.
MATERIALS AND METHODS
Animal preparation
Adult female Sprague-Dawley rats were anaesthetized with Nembutal (80 μl/100 g body weight; Boehringer Ingelheim, Indianopolis, IN) and killed by decapitation, and the olfactory bulbs were dissected and fixed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), pH 7.4, for 24 hours at 4°C. These olfactory bulbs were processed and embedded in paraffin wax. Serial coronal sections (8 μm) from six animals were cut and collected on 2% aminopropyltriethoxysilane-coated slides (Sigma Chemical Corporation, St. Louis, MO). All procedures were carried out with the approval of and in accordance with the University of Queensland Animal Ethics and Experimentation Committee.
Immunohistochemistry
Immunohistochemistry was performed as described previously (Key and Akeson, 1993). Paraffin sections of adult olfactory bulbs were dewaxed, rehydrated, and blocked for 30 minutes with 2% bovine serum albumin BSA with Triton X-100 (TX-100) in Tris-buffered saline (TBS), pH 7.4. The following primary antibodies were used: 2B8, a mouse monoclonal antiserum against NOC-3 (1:1 dilution; Dowsing et al., 1997); a goat polyclonal antiserum against olfactory marker protein (OMP; 1:1,000 dilution; Keller and Margolis, 1975); and KH10, a mouse monoclonal antibody against a lactoseries carbohydrate (1:30 dilution). The KH10 antibody was developed by T.M. Jessel and J. Dodd and was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences at the University of Iowa (Iowa City, IA). Primary antibodies were incubated overnight at 4°C. Sections were then washed with TBS with TX-100, incubated either with biotinylated horse anti-mouse immunoglobulin antibodies (1:200 dilution; Vector Laboratories Inc., Burlingame, CA) or with biotinylated donkey anti-goat immunoglobulin antibodies (1:400 dilution; Dako A/S, Glostrup, Denmark) for 2 hours at room temperature. Sections were then washed and incubated with avidin-biotin-horseradish peroxidase (Vectastain Elite ABC kit; Vector Laboratories Inc.) for 1 hour at room temperature, and staining was visualized by reaction with diaminobenzidine and H2O2 in TBS. Control sections incubated with either 2% BSA with TX-100 in TBS or with preimmune serum produced negligible background staining. Some sections were counterstained with hematoxylin (Vector Laboratories Inc.).
Digital images were collected with a Spot 2 camera (Spot Diagnostic Instruments, Inc., Sterling Heights, MI) fitted on an Olympus BH2 microscope (Olympus, Tokyo, Japan) with differential interference contrast optics. Color levels and contrast of images were balanced and adjusted using Adobe Photoshop software (version 4.0; Adobe Systems Inc., San Jose, CA) without further digital manipulation. Every second serial section through the olfactory bulb was used for three-dimensional reconstruction with Surf Driver software (University of Hawaii and University of Alberta) using the midline and vomeronasal nerve as topographical reference points. The topographical position of a glomerulus along the rostrocaudal axis was calculated using serial sections of both left and right olfactory bulbs from six animals. This absolute position was determined by counting the number of the 8-μm-thick sections between the most rostral section of the olfactory bulb in which glomeruli were first detected (by OMP immunohistochemistry and hematoxylin counterstaining) and the NOC-3+ glomerulus. To standardize for interanimal variation in the size of the olfactory bulb, the relative glomerular position was determined by dividing the absolute position by the total length of the olfactory bulb (from the most rostral end of the main olfactory bulb to the most caudal end of the accessory olfactory bulb). The most rostral end of the main olfactory bulb was defined as the most rostral coronal section in which glomeruli were observed, and the most caudal end of the accessory olfactory bulb was defined as the most caudal section in which the accessory olfactory bulb was observed. The mean relative distances and standard deviations were calculated from the data for both left and right olfactory bulbs from six animals.
RESULTS
Primary olfactory neurons in rat are not a uniform population but, rather, are a mixed population of cells that express different odorant receptors (Buck and Axel, 1991) and cell surface carbohydrates (for reviews, see Key, 1998; Plendl and Sinowatz, 1998). Although odorant receptors are expressed by primary olfactory neurons projecting to the same glomerulus, there is no evidence to date showing that the spatial expression of cell surface carbohydrates is similarly specific to neurons projecting to the same glomeruli. NOC-3 is an uncharacterized carbohydrate epitope that is present on discrete glycoforms of N-CAM expressed by a subpopulation of primary olfactory axons in rat (Dowsing et al., 1997). We set out to determine whether NOC-3 was expressed on axons that target glomeruli in topographically fixed positions in the rat olfactory bulb.
Novel N-CAM glycoform is expressed by subpopulations of primary olfactory neurons
In the adult rat olfactory neuroepithelium, NOC-3 was expressed by subsets of primary olfactory neurons distributed throughout the whole nasal cavity (Fig. 1A). NOC-3 was localized to the perikarya, dendrites, and axons of primary olfactory neurons that were dispersed mosaically among neurons that did not express NOC-3 (Fig. 1B). Thus, NOC-3 was not localized selectively to any one or combination of the four previously described zones in the nasal cavity (Ressler et al., 1993; Vassar et al., 1993). The NOC-3 axons projected to the olfactory bulb, where they terminated in a widely dispersed subpopulation of glomeruli.
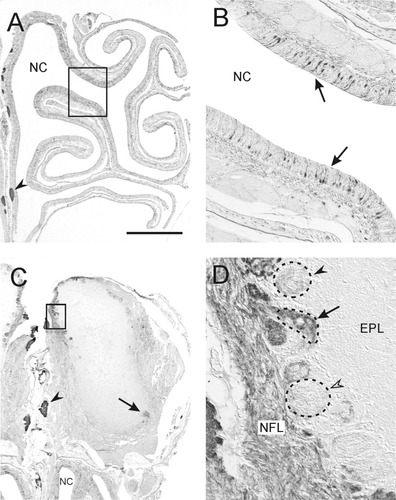
NOC-3 distribution in the adult rat olfactory neuroepithelium and rostral olfactory bulb. A: Low-power photomicrograph of the olfactory neuroepithelium. NOC-3-positive (NOC-3+), primary olfactory neurons were present in all regions of the olfactory epithelium. The boxed area is shown in B. Axon bundles (arrowhead) in the accessory olfactory nerve were strongly immunoreactive for NOC-3. B: Subpopulations of primary olfactory neurons (arrows) were positive for NOC-3 and were dispersed among neurons that were immunonegative. C: In the rostral one-third of the olfactory bulb, NOC-3 was expressed widely, especially in the dorsal olfactory bulb. NOC-3 also was expressed in glomeruli in the ventral olfactory bulb (arrow). The boxed area is shown in D. The accessory olfactory nerve was strongly immunopositive (arrowhead). D: In the dorsal olfactory bulb, NOC-3+ axons were distributed diffusely throughout the nerve fiber layer (NFL) without obvious fasciculation. Some glomeruli were strongly positive for NOC-3 (arrow), whereas others were weakly positive (solid arrowhead) or negative (open arrowhead) for NOC-3. All photomicrographs are coronal views with dorsal to the top. EPL, external plexiform layer; NC, nasal cavity. Scale bar = 1,000 μm in A,C, 200 μm in B, 100 μm in D.
In the rostral olfactory bulb, NOC-3-positive (NOC-3+) glomeruli were localized predominantly to the dorsal surface (Fig. 1C), although some weakly labeled glomeruli were present ventrally (Fig. 1C, arrow). Within the rostrodorsal olfactory bulb, NOC-3+ axons appeared to be dispersed widely in the nerve fiber layer and did not sort out and selectively fasciculate into discrete bundles (Fig. 1D). Some glomeruli were stained strongly by NOC-3 antibodies (Fig. 1D, arrow), whereas other glomeruli exhibited weak or no staining. NOC-3+ glomeruli were interspersed among glomeruli that were immunonegative. In contrast, the accessory olfactory nerve and olfactory bulb were stained strongly and uniformly for NOC-3 (Fig. 1A,C, arrowhead; Fig. 2G). The mosaic distribution of a large number of NOC-3 glomeruli, the variable levels of NOC-3 staining, and the difficulty in obtaining sections at the same rostrocaudal level in different bulbs precluded the reliable identification of the same NOC-3 glomerulus in the rostral half of the olfactory bulb, either between animals or even in different bulbs of the same animal. Although clusters of NOC-3 glomeruli consistently were localized on the dorsorostral surface of the olfactory bulb, it was not always possible to identify the same NOC-3 glomerulus (Fig. 2A,B). In some animals, we were able to tentatively identify NOC-3 glomeruli lying in the same positions relative to one another in the right and left olfactory bulbs (Fig. 2C–F). In the absence of distinct morphological features, however, we were not able to identify these same glomeruli in different animals.
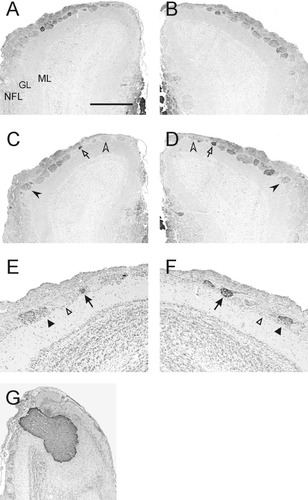
NOC-3 glomeruli in the rostral olfactory bulb. Sections of the left (A,C,E) and right (B,D,F) olfactory bulbs at the same level from three different animals. A,B: A distinct cluster of NOC-3 glomeruli was present in dorsorostral bulb of all animals. In some animals (C,D and E,F), the same glomeruli could be identified tentatively in the right and left olfactory bulbs but not between bulbs in different animals. G: The accessory olfactory nerve and bulb were uniformly immunoreactive for NOC-3. All photomicrographs are coronal views with dorsal to the top. Sections E–G were counterstained with hematoxylin. GL, glomerular layer; ML, mitral cell layer; NFL, nerve fiber layer. Scale bar = 500 μm in G, 400 μm in A–D, 200 μm in E,F.
NOC-3 is expressed by specific glomeruli in topographically fixed positions
Although the same NOC-3 glomeruli could not be identified reliably in the rostral olfactory bulb between animals, this was not the case in the caudal olfactory bulb. Three glomeruli were distinguished easily by a combination of their strong NOC-3 staining and their distinct morphological features. About midway along the rostrocaudal length of the olfactory bulb, a relatively large and prominent glomerulus (Fig. 3A, arrow) was strongly immunopositive for NOC-3. This glomerulus appeared to be in the same position in both the left and right olfactory bulbs. In sections rostral to this glomerulus, small fascicles of NOC-3 axons were dispersed in the nerve fiber layer (Fig. 3B). These axons coalesced into large bundles before terminating in this large lateral glomerulus (Fig. 3C,D). Serial section analysis revealed that the middle of the lateral glomerulus had three distinct subcompartments separated by a thin layer of periglomerular cells (Fig. 3D). The whole glomerulus was encased by a thick shell of periglomerular cells (Fig. 3D). In the more caudal portions of the glomerulus, the subcompartments merged into a single large, structure (Fig. 3E,F). To determine whether the topographical position of the ventrolateral glomerulus was fixed, we mapped its spatial position along the rostrocaudal axis in six animals. Because the size of the olfactory bulb in adult rats varies between animals, depending on their weight and age, we normalized the position relative to the overall length of the bulb. The mean length of the lateral glomerulus was 410 μm, which was ≈10% of the length of the olfactory bulb. The rostral and caudal ends of this glomerulus were found at 54% ± 6% and 64% ± 6%, respectively, along the length of the rostrocaudal axis of the olfactory bulb (Table 1, Fig. 4). The small variation in the position of this glomerulus indicates that the rostrocaudal location of the glomerulus is highly conserved. In fact, due to the peculiar morphology of this large, lateral glomerulus and its consistent position (Fig. 4), it is possible to identify the glomerulus even without immunostaining.
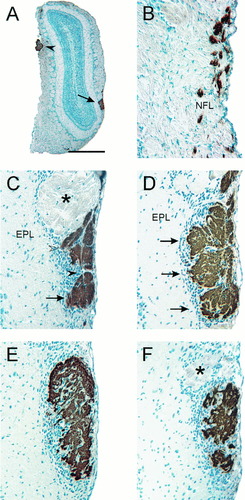
Morphological identification of the lateral glomerulus. A: Low-power photomicrograph demonstrating the topographically consistent position of the glomerulus (arrow) in the lateral olfactory bulb. The accessory olfactory nerve is indicated (arrowhead). B: In the nerve fiber layer (NFL) rostral to the glomerulus subpopulations of axons were strongly immunoreactive for NOC-3 and were tightly fasciculated in dense bundles. Other bundles of axons were negative for NOC-3. C–F show the same glomerulus, with C the most rostral section and F the most caudal section. C: At the rostral end of the lateral glomerulus, the bundles of NOC-3+ axons coalesced into large bundles (open arrowhead), and the glomerulus (arrow) was slightly more ventral to the bundles of axons. Periglomerular cells (solid arrowhead) surrounded the glomerulus. An adjacent glomerulus had low immunoreactivity for NOC-3 (asterisk). D: The more rostral portion of the glomerulus exhibited compartmentalization with three sections (arrows) that were each partially surrounded by periglomerular cells. E: Toward the caudal portion of the glomerulus, the subcompartmentalization disappeared, and the glomerulus had the appearance of a single structure. F: The most caudal portion of the glomerulus had a fragmented appearance, with many periglomerular cells interspersed amongst the NOC-3+ axons. A glomerulus that was weakly immunoreactive for NOC-3 is shown (asterisk). All photomicrographs are coronal views with dorsal to the top, and all sections were counterstained with hematoxylin. EPL, external plexiform layer. Scale bar = 1,000 μm in A, 100 μm in B–F.
| Glomerulus | Position | |
|---|---|---|
| Rostral end | Caudal end | |
| Lateral | 0.54 ± 0.06 | 0.64 ± 0.06 |
| Ventrolateral | 0.67 ± 0.07 | 0.76 ± 0.10 |
| ventromedial | 0.86 ± 0.08 | 0.94 ± 0.07 |
- 1 Data are presented as the ratio of the distance of the rostral or caudal end of the glomerulus from the rostral end of the olfactory bulb compared with the total length of the olfactory bulb.
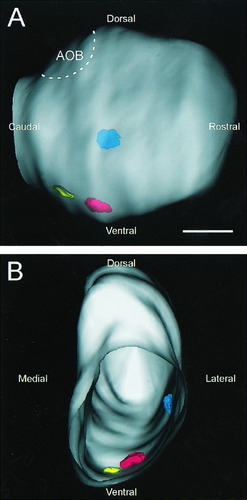
Three-dimensional reconstruction of the large NOC-3+ glomeruli. A: In a view of the lateral surface of the left olfactory bulb, the three large glomeruli that strongly express NOC-3 can be seen: the lateral glomerulus (blue), the ventrolateral glomerulus (red), and the ventromedial glomerulus (yellow). These glomeruli are located in topographically fixed positions that are consistent between the left and right olfactory bulbs and between animals. Rostral is to the right, and dorsal is to the top. B: A perspective of the same olfactory bulb shown in A but viewed from the caudal end of the olfactory bulb. The bulb is see as though it was hollowed out, leaving only the outer surface of the nerve fiber layer and the three glomeruli, which are visible in their fixed topographical positions. Dorsal is to the top, and lateral is to the right. AOB, accessory olfactory bulb. Scale bar = 1,000 μm.
At 67% along the rostrocaudal length of the olfactory bulb, we observed another unusually large glomerulus that was strongly NOC-3+ (Fig. 5A). This glomerulus, located in the ventral midline, was surrounded by glomeruli that were not stained for NOC-3 (Fig. 5C). Similar to the lateral glomerulus, small bundles of axons that expressed NOC-3 rostral to the glomerulus were tightly fasciculated and were interspersed among fascicles that did not express NOC-3 (Fig 5B). The glomerulus was elongated along its ventral edge and was subcompartmentalized like the lateral glomerulus, but only in its caudal portions (Fig. 5D). The ventrolateral glomerulus was also an unusually long glomerulus and extended for ≈10% of the length of the olfactory bulb (Table 1, Fig. 4).
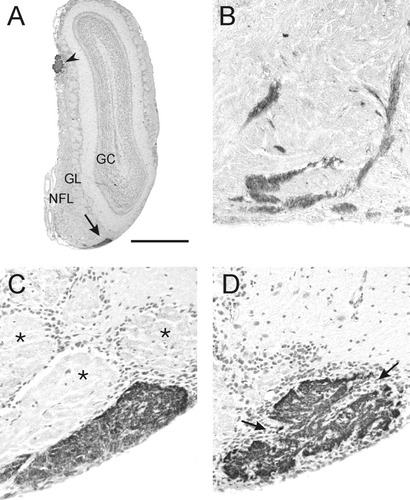
Morphological identification of the ventrolateral glomerulus. A: Low-power photomicrograph of olfactory bulb demonstrating the ventral position of the ventrolateral glomerulus (arrow). The accessory olfactory nerve is indicated (arrowhead). B–D are in the same ventral position, with B the most rostral section and D the most caudal section. B: Rostral to the glomerulus, axon bundles that were NOC-3+ were tightly fasciculated in dense bundles and were interspersed among other bundles of axons that were immunonegative. C: The rostral portion of the glomerulus was a single, large structure that was elongated along the ventral surface of the olfactory bulb. Glomeruli adjacent to the NOC-3 glomerulus were completely immunonegative (asterisks). D: Subcompartmentalization of the glomerulus is characteristic of the caudal portion of the glomerulus, and periglomerular cells (arrows) were present in the areas between the compartments. All photomicrographs are coronal views with dorsal to the top, and all sections were counterstained with hematoxylin. GC, granule cell layer; GL, glomerular layer; NFL, nerve fiber layer. Scale bar = 1000 μm in A, 100 μm in B–D.
Toward the caudal end of the olfactory bulb, there were several glomeruli that were NOC-3 immunoreactive. These glomeruli were found on the dorsal, lateral, and ventral surfaces of the olfactory bulb (Fig. 6A). Only the ventral glomerulus was relatively large and resembled the two more rostral large glomeruli. This large ventrocaudal glomerulus typically contained four distinct subcompartments (Fig. 6B,C) and extended for 8% of the length of the olfactory bulb (Table 1, Fig. 4). These subcompartments merged in the most caudal portion of the glomerulus (Fig. 6D).
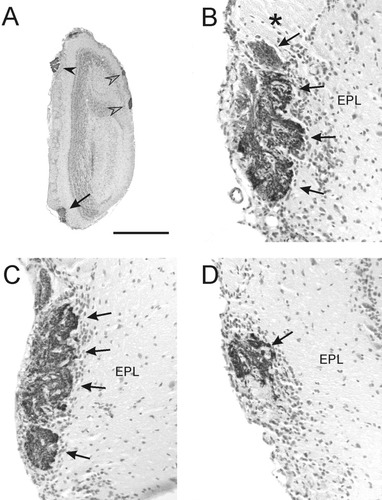
Morphological identification of the ventromedial glomerulus. A: Low-power photomicrograph of an olfactory bulb demonstrating the ventral-medial position of the ventromedial glomerulus (arrow). NOC-3+ glomeruli in the dorsal and lateral bulb are indicated (open arrowheads), as is the accessory olfactory nerve (solid arrowhead). B–D are in the same medial position, with B the most rostral section and D the most caudal section. B: This large glomerulus exhibited considerable sub compartmentalization in the rostral portion of the glomerulus, with four obvious subcompartments (arrows). An adjacent glomerulus (asterisk) was immunonegative. C: These subcompartments (arrows) persisted throughout the glomerulus. D: In the most caudal portion of the glomerulus, only part of the glomerulus was positive for NOC-3 (arrow). All photomicrographs are coronal views with dorsal to the top, and all sections were counterstained with hematoxylin. EPL, external plexiform layer. Scale bar = 1,000 μm in A, 100 μm in B–D.
Large NOC-3+ glomeruli are a unique subset of glomeruli
Previous studies have revealed the presence of a group of interconnected glomeruli circling the caudal olfactory bulb (Shinoda et al., 1989). These glomeruli are identified by their weak immunoreactivity with anti-OMP antibodies and by strong immunostaining for the lactoseries carbohydrate KH10 (Ring et al., 1997). To determine whether the large NOC-3+ glomeruli were necklace glomeruli, we immunostained adjacent serial sections for NOC-3, OMP, and KH10. All three large NOC-3+ glomeruli exhibited weak expression of OMP, whereas adjacent glomeruli strongly expressed OMP (for data on the lateral glomerulus, see Fig. 7A). The NOC-3+ glomeruli also were immunopositive for the lactoseries carbohydrate KH10, whereas adjacent glomeruli were not (Fig. 7B).
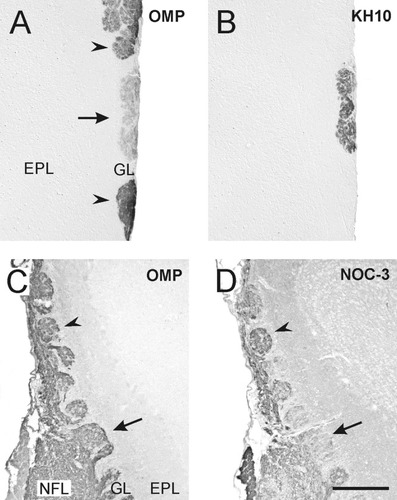
The glomeruli are necklace glomeruli. A: The lateral glomerulus (arrow) was weakly immunoreactive for olfactory marker protein (OMP) compared with surrounding glomeruli (arrowheads) that were strongly immunoreactive. B: The lateral glomerulus was strongly immunoreactive for the antibody KH10, whereas surrounding glomeruli were immunonegative. C: In the dorsal region of the rostral olfactory bulb, all glomeruli exhibited equivalent OMP immunoreactivity. D: In a section adjacent to C, some glomeruli exhibited strong expression of NOC-3 (arrowhead), whereas other glomeruli exhibited weaker expression of NOC-3 (arrow): compare with labeled glomeruli in C. GL, glomerular layer; EPL, external plexiform layer; NFL, nerve fiber layer. Scale bar = 100 μm in A,B, 200 μm in C,D.
Not all glomeruli that expressed NOC-3 had characteristics typical of the necklace glomeruli. Glomeruli in the dorsal portion of the rostral olfactory bulb all were equally immunopositive for OMP (Fig. 7C) but exhibited varying immunoreactivity for NOC-3 (Fig. 7D). Thus, NOC-3 immunostaining is not a specific marker of necklace glomeruli. In fact, it appears that NOC-3 staining of the three large glomeruli in the olfactory bulb detects a unique subpopulation of necklace glomeruli.
DISCUSSION
It has been proposed by several groups that carbohydrates may be involved in the selective fasciculation and targeting of primary olfactory axons (Schwarting et al., 1992; Mahanthappa et al., 1994; Gong and Shipley, 1996; Puche and Key, 1996; Saito et al., 1999; St. John and Key, 1999); however, direct functional evidence is lacking. Part of the problem in determining the role of cell surface carbohydrates is that, typically, they are expressed by large subpopulations of primary olfactory neurons that project to many widely dispersed glomeruli in the olfactory bulb. For example, due to the large number of NOC-3+ glomeruli in the rostral olfactory bulb, and because their morphology was essentially identical to other glomeruli in that region, it was not possible to accurately map the same glomerulus in different bulbs.
We took advantage of the fact that antibodies against NOC-3 label three morphologically unique and large glomeruli in the central and caudal regions of the olfactory bulb to address the question of whether axons that express the same carbohydrate converge and consistently target the same glomerulus. We have shown here that the three glomeruli are in topographically fixed positions in both the left bulb and the right bulb of the same animal as well as in different animals. At least one of these glomeruli can be identified readily, even without NOC-3 immunostaining, on the basis of its large size and its position on the lateral surface of the bulb. Thus, it is quite clear that axons expressing the same cell surface carbohydrates are able to sort out and target specific glomeruli in the olfactory bulb.
Primary olfactory neurons that express particular odorant receptors project their axons to glomeruli that are in topographically fixed positions in each olfactory bulb (Vassar et al., 1993, 1994; Ressler et al., 1994; Mombaerts et al., 1996; Royal and Key, 1999). In the present study, we have show for at least some glomeruli innervated by axons expressing NOC-3 that they also are in topographically fixed positions in the olfactory bulb. Together, these results indicate that axons innervating the same glomerulus are identified uniquely both by their expression of odorant receptor type and by their cell surface carbohydrates.
The mechanisms of axon convergence to specific glomeruli are not fully understood. The odorant receptor is likely to be involved, although, by itself, it is not sufficient to account completely for targeting (Mombaerts et al., 1996; Wang et al., 1998). The presence of cell surface carbohydrates on olfactory axons is not necessarily an indication that they are involved in selective fasciculation or axon targeting. However, when we consider that axon targeting is disrupted in mice deficient in galectin-1 (Puche et al., 1996), a carbohydrate-binding protein, and that axons expressing the same cell surface carbohydrate innervate the same glomerulus, then interactions between carbohydrates and their binding proteins probably have a role in axon growth and guidance in the olfactory nerve pathway.
Cell surface carbohydrates may form a glycocode that is involved in the sorting and targeting of axons. This code may consist of different carbohydrates and/or the same carbohydrate expressed in different amounts by different subpopulations of axons. How do carbohydrates play a role if they are widely expressed by axons arising from many zones in the olfactory epithelium? We believe that axons arising from different zones probably are partitioned by the expression of zonal/regional-specific cell adhesion molecules, such as O-CAM (Yoshihara et al., 1997). Carbohydrates may then play a role in the sorting of axons in each one of these regional subdivisions. The coding we have shown for NOC-3 is only one potential marker. Many other subpopulations become apparent when the expression patterns of multiple carbohydrates are examined (Dowsing et al., 1997). It is possible that the NOC-3+ axons innervating each of the three large glomeruli are identified uniquely by the expression of other combinations of carbohydrates, just as they probably are encoded uniquely by odorant receptor expression. Next, we plan to test whether NOC-3 is involved in axon sorting and targeting by genetically altering the expression of carbohydrates by primary olfactory neurons.
The three large glomeruli have a consistent morphological appearance and typically exhibit subcompartments. It is unknown at present whether the subcompartments of the large glomeruli have a specific functional role or whether they are only a consequence of their large morphology. It is possible that, in fact, the different subcompartments are innervated by neurons expressing different odorant receptors. Whereas the three large glomeruli have previously been shown to be part of the necklace glomeruli (Ring et al., 1997), we have demonstrated that at least some of them can be identified by the expression of the NOC-3 glycoform of N-CAM. Thus, these large glomeruli constitute a unique subset that has not been identified previously.
Acknowledgements
This work was supported by grants from the National Health and Medical Research Council to J.St.J. and B.K. We thank Linh Nguyen for the excellent technical assistance with immunohistochemistry.




