Normal chiasmatic routing of uncrossed projections from the ventrotemporal retina in albino Xenopus frogs
Abstract
Albino mammals lacking melanin in the embryonic retinal pigment epithelium (RPE) have abnormal retinal decussation patterns at the optic chiasm: their uncrossed projections are smaller and arise from fewer, more peripheral temporal retinal ganglion cells than in con-specific wild-types. To determine whether these abnormalities generalize to nonmammalian mutants, we used anterograde and retrograde labeling methods to compare the distribution of retinal projections to the thalamus in adult normal and albino Xenopus frogs. In both pigmentation phenotypes, crossed retinal terminations covered ∼80% of the neuropil of Bellonci (nB) and corpus geniculatum thalamicum (cgt) and uncrossed inputs occupied, respectively, approximately 75% and 25% of these two main visual centers. In the wild-type frogs and in the albinos, ganglion cells giving rise to the crossed projections were distributed throughout the retina, whereas ipsilaterally projecting cells were confined to a specific ventrotemporal retinal division. This region comprised ∼40% of the total retinal area, was bordered by a well-defined line of decussation, and contained an average of ∼3,000 ipsilaterally projecting ganglion cells of equivalent soma sizes in the two pigmentation phenotypes. In summary, we found no evidence of chiasmatic misrouting in the uncrossed retinothalamic projections of albino Xenopus, even though these pathways are substantial in normal frogs and share features in common with mammalian retinogeniculate projections. Our findings suggest that congenital RPE melanin deficiency results in major defects in the development of the retina and its central projections only in mammals. J. Comp. Neurol. 458:425–439, 2003. © 2003 Wiley-Liss, Inc.
The optic chiasm is a key decision point in the developing retinal pathways where ganglion cell axons can cross the midline or take an uncrossed route. A major factor governing this choice is the ganglion cells' position in the retina. In general, only axons belonging to temporal or to ventrotemporal ganglion cells proceed ipsilaterally, their precise retinal origin being dependent on the size and location of the animals' binocular field of view. To explain this distinctive behavior, Sperry (1963) proposed that uncrossed ganglion cells acquire special properties (e.g., chemo-specificities) through exposure to positional information in the embryonic retina that prevents their axons crossing the midline. Recent experimental studies of normal chiasm formation are consistent with this theoretical position. For example, real-time video recordings and static analyses of dye-labeled retinal axons in vivo (Godement et al., 1990, 1994; Marcus et al., 1995; Nakagawa et al., 2000) and in vitro assays (Wizenmann et al., 1993; Wang et al., 1995) have demonstrated that, although nasal axons are capable of extension over chiasmatic cells and their membranes, temporal axon growth is actively inhibited and repelled by encounters with the very same tissues. The mechanisms that specify uncrossed ganglion cells to avoid the midline, thus, are critical determinants of retinal decussation patterns at the optic chiasm.
Albino and other hypopigmented mammals congenitally lacking ocular melanin have provided important insights into these processes (for reviews, see Guillery, 1986; Jeffery, 1997). In all such mutants, uncrossed retinal projections to the lateral geniculate nucleus (LGN) and other visual centers of the thalamus and midbrain are markedly reduced in size compared with con-specific wild-types. This defect occurs because many developing ganglion cells of the temporal retina that should project ipsilaterally misroute their axons across the chiasm to the opposite side of the brain (Bunt et al., 1983; Cucchiaro and Guillery, 1984; Kliot and Shatz, 1985; Chan and Guillery, 1993; Marcus et al., 1996). Misrouting particularly affects ganglion cells that are normally born early in development and destined to occupy the nasotemporal division (Dräger, 1985a; Reese et al., 1992; Baker and Reese, 1993). As a consequence, this division of the mutant retina becomes shifted toward the temporal periphery and does not correspond to the vertical meridian of the visual field, as in normally pigmented mammals (e.g., rodents: Lund, 1965; Creel and Giolli, 1972; LaVail et al., 1978; carnivores: Guillery, 1969; Sanderson et al., 1974; Stone et al., 1978b; Leventhal and Creel, 1985; Morgan et al., 1987; humans: Creel et al., 1974; Guillery et al., 1975; Morland et al., 2002). Moreover, the severity of these abnormalities has been shown to correlate with the degree of melanin deficiency in the embryonic RPE of various hypopigmentation phenotypes, regardless of the underlying gene mutation or of its effects on other ocular tissues (e.g., mice: LaVail et al., 1978; Balkema and Dräger, 1990; mink: Sanderson et al., 1974; Siamese vs. albino cats: Stone et al., 1978b; Cooper and Pettigrew, 1979; Leventhal and Creel, 1985; humans: Oetting and King, 1999).
These findings clearly implicate the RPE as a source of positional information for specifying uncrossed chiasmatic pathway choice, and they suggest that early melanin expression influences the process in a dose-dependent manner. The intercellular signaling mechanisms involved remain unknown, but Dräger (1985b) has proposed that gap junctions established between the RPE and ganglion cell precursors in the embryonic mammalian retina (Townes-Anderson and Raviola, 1981) could provide the anatomic substrate. Indeed, Dräger (1985b) has shown that melanin normally acts as a powerful calcium buffer in the developing RPE, and she suggests that the reduced Ca2+-availability in these cells of the hypopigmented retina could disrupt gap junctional opening times, so altering the signals transmitted to early-born ganglion cells.
Precocious RPE melanin synthesis and gap junction coupling are conserved features of the embryonic vertebrate retina (e.g., Xenopus: Dixon and Cronly-Dillon, 1974; Hayes, 1976), so this model predicts that chiasmatic misrouting should also occur among nonmammalian albinos. Surprisingly, this possibility has only ever been examined twice before and with conflicting outcomes. Guillery and Updyke (1976) found no abnormalities in albino axolotls, whereas Dunn-Meynell et al. (1983) showed that an uncrossed retinal projection to the optic tectum is reduced or absent in albino channel catfish. We have re-investigated this issue, by comparing the origin and termination of crossed and uncrossed retinal projections to the thalamus in adult normal and albino Xenopus frogs. We selected this species because wild-type adults are known to possess an extensive frontosuperior binocular field (Grant and Keating, 1986) and robust uncrossed projections to thalamic targets analogous to the mammalian LGN originating mainly from the ventrotemporal retina (Levine, 1980; Kennard, 1981; Hoskins and Grobstein, 1985a). Misrouting, thus, should be readily detectable in the albino phenotype (Hoperskaya, 1975).
MATERIALS AND METHODS
The Xenopus frogs were adult males obtained from commercial suppliers, the wild-types from Blades Biological (Edenbridge, Kent, UK) and the albinos from Xenopus 1 (Ann Arbor, MI). Animals were 5–7 years old and of similar size and weight at the time of injection (see Table 2). All procedures were conducted with United Kingdom Home Office approval and in accordance with their regulations. Albinos were homozygous for the “periodic” tyrosinase gene mutation first described by Hoperskaya (1975) and had no obvious macroscopic pigmentation of the eyes or skin. To evaluate this histologically, the heads of three albino frogs were paraffin-embedded and serially sectioned at 10 μm in the horizontal plane, and the eyes from three others were plastic-embedded and sectioned at the same thickness along the nasotemporal or dorsoventral axes. Sections were stained with hematoxylin and examined microscopically for melanin pigment granules.
| Animals Code | wt (g) | NA (mm) | Injection sites | |||
|---|---|---|---|---|---|---|
| Survival (day) | Size | Location | Main involvement(s) | |||
| Wild-types | ||||||
| WT 10 | 58 | 81 | 6 | Large | Lateral | nB, cgt, mOT |
| WT 11 | 56 | 80 | 8 | Medium | Medial | LGN, nB, cgt |
| WT 15 | 55 | 76 | 6 | Medium | Lateral | nB, cgt (mOT) |
| WT 17 | 52 | 74 | 7 | Large | Lateral | nB, cgt, mOT |
| WT 19 | 51 | 75 | 7 | Small | Very lateral | mOT (nB) |
| Albinos | ||||||
| ALB 16 | 49 | 75 | 6 | Small | Very lateral | mOT (nB) |
| ALB 20 | 42 | 70 | 6 | Large | Lateral | nB, cgt, mOT |
| ALB 22 | 48 | 74 | 6 | Large | Lateral | nB, cgt, mOT |
| ALB 23 | 43 | 72 | 7 | Medium | Lateral | nB, cgt (mOT) |
| ALB 24 | 38 | 63 | 7 | Large | Medial | LGN, nB, cgt |
- 1 NA, nasoanal length; nB, neuropil of Bellonci; cgt, corpus geniculatum thalamicum; mOT, marginal optic tract; LGN, lateral geniculate nucleus.
Anterograde tracer injections
Frogs of each pigmentation phenotype were injected in one eye with [3H]proline to trace the crossed and uncrossed retinal projections to the thalamus. Animals were anesthetized by immersion in a 1:100 solution of MS222 (ethyl-m-aminobenzoate; Sigma). A small puncture was made at the sclerocorneal junction in the dorsolateral part of one eye by using a 26-guage needle, and 1–2 μl of [3H]proline (5 μCi/μl; Amersham, UK) was pressure injected into the vitreous chamber. Frogs were allowed to survive for 1–2 days after injection, when they were given a lethal dose of MS222 (25 mg in 10% solution, i.p.), decapitated, and their heads placed in Carnoy's fixative. Brains were dissected free and embedded in paraffin, and serial 10-μm transverse sections were mounted on gelatinized slides and processed for autoradiography. Slides were dipped in emulsion (Kodak NTB-2-Nuclear), exposed for 21 days, developed, and counterstained with hematoxylin.
Analysis of retinal terminal labeling
The overall distribution of [3H]proline label in the optic pathways and terminal zones of the thalamus, contralateral and ipsilateral to the injected eye, was charted by camera lucida, on drawings (40× magnification) of selected sections taken at regular intervals (1 in 5) through each brain. The regions of interest in this analysis were the nB, cgt, and rostral visual nucleus (rvn), all of which are known to receive direct retinal inputs from both eyes in normal adult Xenopus (Levine, 1980; Kennard, 1981; Hoskins and Grobstein, 1985a). To estimate the extent of the retinal input to these targets, their outlines in each section were redrawn at higher magnification (250×) and the region(s) within them containing fine-grain terminal label were plotted on the enlarged drawings. The total area of each target structure and its labeled region(s) were measured by using a drawing tablet linked to a scanning program (Sigma-Scan; Jandel Scientific). By adding the data obtained from each section and factoring in the section thickness and interval examined (i.e., 50× μm), the approximate volumes of the three target zones and their relative proportions occupied by crossed or uncrossed terminal inputs were calculated.
Retrograde tracer injections
To compare the number and distribution of retinal ganglion cells giving rise to these projections, retrograde tracer was injected unilaterally into the anterior thalamus. Animals were anesthetized by injection of MS222 (0.25 mg/g in 10% solution, i.p.). The cranium overlying the thalamus and midbrain tectum was removed, and the dura and arachnoid membranes covering the dorsal surface of the diencephalon were retracted. By using a micromanipulator, a fine-barreled pipette attached to a 1-μl Hamilton syringe was inserted into the lateral region containing the nB and cgt, and 0.25–0.5 μl of the lipophilic fluorescent dye 4-(4-didecylaminostyrl)-N-methylpyridinium iodide (4-10-Di-ASP; Molecular Probes, Eugene, OR), dissolved to a concentration of 5–10% in absolute alcohol, was pressure injected into the brain. The cranial bone flap was replaced and the surrounding cavity packed with sterile gelfoam. Cutaneous wounds were sutured, and local anesthetic gel was applied. Frogs were returned to their home containers for 6–8 days, then overdosed with MS222 (as above). Their heads were placed in fixative (4% paraformaldehyde in 0.1 M PBS, pH 7.2). The cornea and lens was removed from each eye 2 hours after initial fixation to enhance penetration within the globe. Twelve to18 hours later, the retinae were dissected out, stripped of as much RPE as possible, and flat-mounted onto plain slides, ganglion cell layer up. The whole-mounts were covered with glycerol:PBS medium (3:7 mixture) and mounted semipermanently under a coverslip sealed with a ring of nail-varnish.
To reconstruct the injection sites, brains were removed from the skull, post-fixed for at least 24 hours, embedded in 4% agar, and Vibratome sectioned in the transverse plane at 100 μm. Alternate sections were stained with hematoxylin or mounted semipermanently (as above) for fluorescence microscopy. The Nissl-stained sections of the thalamus were drawn at 40×, and key structures such as the marginal optic tract (mOT), nB, and cgt, and medial cell groups—including what has been termed the “lateral geniculate nucleus” (Scalia and Gregory, 1970)—were plotted on the drawings, along with the main dye deposit. The extent of diffusion from this site was then added to the drawings, from examination of adjacent sections under the fluorescein isothiocyanate (FITC) filter of a fluorescence microscope.
Analysis of retinal ganglion cell labeling
Whole-mounted retinae were drawn at a magnification of 40× by using camera lucida. Recorded on these drawings were the positions of the optic nerve head and the major choroidal blood vessel, which are situated, respectively, in the dorsonasal quadrant and at the ventral pole of the retina in adult Xenopus (Grant and Keating, 1986). Also included were details of the remaining vascular network, any residual patches of pigment epithelium and other features that could be used as landmarks for orientation purposes.
The distribution of 4-Di-10-ASP–labeled ganglion cells in each retina was plotted by using confocal microscopy (Zeiss Micro Systems, laser scan microscope 410). Microscope settings were adjusted so that picture frames of 319.5 μm × 319.5 μm, corresponding to an area of 0.1 mm2, could be examined by optical sectioning in FITC mode. Retinal areas were selected for analysis by orienting the field of view in light transmission mode with particular landmarks. A series of consecutive 1-μm optical sections was then taken through the ganglion cell layer, and these sections were superimposed to create a two-dimensional composite image. A transparent grid was placed over the computer image and separate counts of the number of labeled cells were performed in either the x- or y-axis of the grid. Between 60 and 80 reconstructed picture frames were created from each retina, resulting in the analysis of some 20–25% of its total surface area. To increase the sampling, manual cell-counts were undertaken of additional regions in each retina by means of an eyepiece grid on the fluorescence microscope, set to cover an area of 0.1 mm2. In this way, counts were obtained for at least 33% of the contralateral cell labeling and 75–100% of ipsilaterally labeled cells.
The cell count for each retinal area was recorded on the drawing at the appropriate location and, by defining the area of maximal labeling as the 100 percentile, isodensity gradient maps were constructed, by using boundaries of 100–75%, 75–50%, 50–25%, 25–1%, and 0% for other regions. The total number of labeled ganglion cells in each retina was then estimated by determining the average cell number/0.1 mm2 in each isodensity region, multiplied by the surface area of the region measured on the drawing tablet. Variable errors in estimating the number of labeled ganglion cells, by the same or by different observers, were within 0–10% of the two means, both for individual retinal regions and for total cell numbers.
RESULTS
Characterization of the periodic albino (ap) phenotype
The mutant frogs displayed phenotypic characteristics of oculocutaneous albinism, with pink eyes and cream-colored skin, contrasting with the black eyes and variable skin pigmentation of the wild-type animals. Histologic examination of sectioned albino eyes and whole-heads confirmed the absence of iridiochoroidal and cutaneous pigment but revealed small amounts of melanin in some cells of the RPE (Fig. 1). To analyze this finding further, the distribution of these hypopigmented cells was charted through the serially sectioned retinae. In all cases, the melanin was confined to a region of the pigment epithelium near to and surrounding the optic nerve head, where retinal cells are generated between middle and late larval life (Straznicky and Gaze, 1971; Grant and Keating, 1986). This pattern of deposition indicates that a partial expression of the tyrosinase gene had occurred only during this developmental phase, a feature of the least pigmented frogs carrying the periodic mutation (Hoperskaya, 1975).
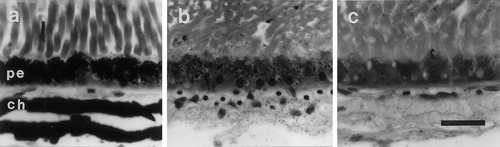
Comparisons of melanin pigmentation in the eyes of (a) wild-type and (b,c) periodic albino (ap) Xenopus frogs. The inner retina is uppermost in each photomicrograph, with the retinal pigment epithelium (pe) and choroid (ch) layers shown in alignment. Both layers are generally devoid of pigment in the albino (c), but some melanin granules are present in the retinal pigment epithelium cells generated during larval life (b). Note that this pigment is greatly reduced compared with the normal levels in a. Note also that the dark-staining blobs evident in the ch layers in b and c are the nuclei of erythrocytes and other cells. Scale bar = 20 μm in a (applies to a–c).
Retinal terminations in the contralateral and ipsilateral thalamus
Crossed and uncrossed retinal projections to the thalamus were examined in 10 normal and 6 albino frogs injected intraocularly with [3H]proline. The general appearance and distribution of retinal fiber and terminal label was similar in all animals, regardless of pigmentation phenotype. As shown in Figures 2-4, substantial retinal projections were observed on both sides of each brain in the largest of the optic terminal centers, the nB, with further bilateral inputs to the underlying cgt and the much smaller rvn. In agreement with previous anterograde transport studies in normal adult Xenopus (Levine, 1980; Kennard, 1981), projections to these thalamic targets contralateral to the injected eye were generally more extensive and denser than those to the equivalent centers on the ipsilateral side. Indeed, nearly all of the contralateral nB and cgt, and much of the rvn, were uniformly filled with fine-grain terminal label at all rostrocaudal levels of each brain, whereas the ipsilateral innervation of these zones exhibited more regional variation (Figs. 2-4). This finding was most evident in the ipsilateral input to nB. At the rostral end, this input appeared equivalent in extent and density to that of the crossed projection to the same territory, whereas at mid-levels it was mainly concentrated as an outer ring, and became confined to the medial half toward the caudal pole. Ipsilateral inputs were notably sparser at all rostrocaudal levels of the two other terminal zones, appearing as just a few weak patches in dorsal regions of the cgt and confined mainly to the ventrolateral sector of the rvn. Importantly, however, none of these features of the uncrossed retinal projections were obviously reduced in the albino frogs.
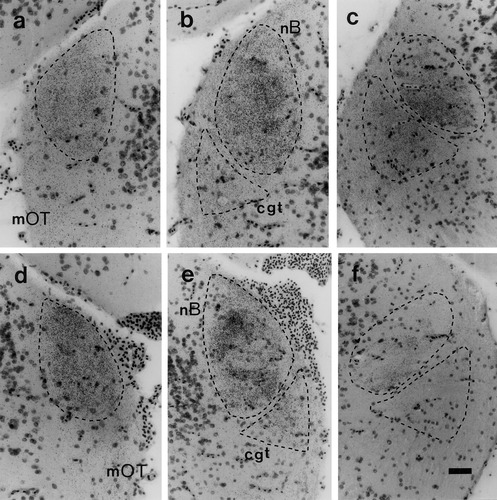
Distribution of [3H]proline label in the crossed (a–c) and uncrossed (d–f) retinal inputs to the neuropil of Bellonci (nB) and the corpus geniculatum thalamicum (cgt) in wild-type Xenopus. The photomicrographs are taken from equivalent regions of the brain at different levels through these optic terminal centers, the extents of which are shown in outline. Dorsal is uppermost in all panels; in a–c, lateral is to the left; in d–f, lateral is to the right. The nB approximates an ellipse, with its rostral end (a, d) lying against the pial surface, its body (b,e) adjoining the marginal optic tract (mOT), and its caudal end (c,f) directed medially. The cgt is a wedge-shaped terminal zone situated just below the nB, except at rostral levels (a,d) to which it does not extend and is bounded laterally and ventrally by marginal and axial optic tract fibers. Fine-grain terminal label fills most of these two targets contralateral to the injected eye. Ipsilateral terminal label is also extensive at rostral-mid levels through the nB but is notably sparse in the cgt. Scale bar = 50 μm in a (applies to a–f).
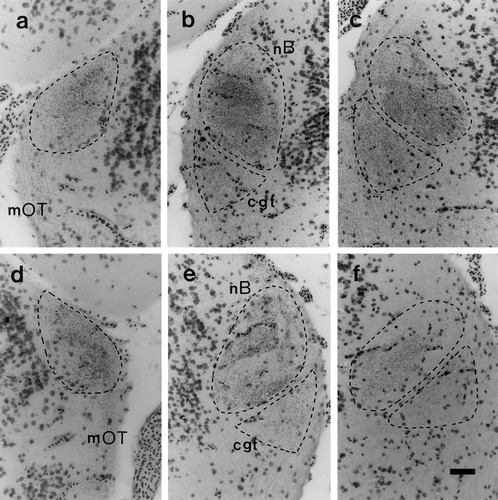
Distribution of [3H]proline label in the crossed (a–c) and uncrossed (d–f) retinal inputs to the neuropil of Bellonci (nB) and the corpus geniculatum thalamicum (cgt) in albino Xenopus. All conventions, as in Figure 2.
Distribution of [3H]proline label in the crossed (a,b) and uncrossed (c,d) retinal inputs to the rostral visual nucleus (rvn) in wild-type (a,c) and albino (b,d) Xenopus. The photomicrographs are taken from equivalent mid-levels of the nucleus. Dorsal is uppermost in all figures; lateral is to the left in a,b and to the right in c,d. The rvn is a very small, roughly spherical mass of cells (as outlined), located beside the ascending marginal optic tract (mOT). In both phenotypes, fine-grain terminal label is present through most of the nucleus contralateral to the injected eye, whereas ipsilaterally the terminations are restricted to its ventrolateral region. Scale bar = 50 μm in a (applies to a–d).
To evaluate this finding quantitatively, the outlines of each target zone on both sides of the brain and of the regions within them containing terminal label were measured at regular intervals spanning their entire rostrocaudal extents, so encompassing regional variations in some of the ipsilateral termination patterns. The data are presented in Table 1 and illustrated in Figure 5. Representative brains from each group of frog were selected for this purpose, in which a complete series of autoradiographic sections through the thalamus had been successfully obtained. Estimates of the total volume of matched terminal zones on opposite sides of the same brain were generally similar, the slight between-animal variability probably being due to differential brain shrinkage. Expressing the labeled region of each zone as a percentage of its total volume (Fig. 5), confirmed that crossed retinal terminations are more extensive in the nB (∼80%), cgt (∼80%), and rvn (∼55%) than the uncrossed input to the same centers (which occupied ∼70%, 25%, and 40%, respectively), and demonstrated no differences between the wild-type and albino frogs in any of these respects.
| Frogs | Neuropil of Bellonci | Corpus geniculatum thalamicum | Rostral visual nucleus | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contralateral | Ipsilateral | Contralateral | Ipsilateral | Contralateral | Ipsilateral | |||||||||||||
| Total | Label | % | Total | Label | % | Total | Label | % | Total | Label | % | Total | Label | % | Total | Label | % | |
| Wild-types | ||||||||||||||||||
| WT 1 | 17.64 | 15.31 | 87 | 16.64 | 11.25 | 68 | 7.56 | 6.56 | 87 | 7.48 | 1.55 | 21 | 2.64 | 1.48 | 56 | 2.52 | 0.98 | 39 |
| WT 3 | 17.95 | 14.25 | 79 | 16.87 | 10.81 | 64 | 6.98 | 6.09 | 87 | 6.89 | 1.35 | 20 | 3.08 | 1.87 | 61 | 3.26 | 1.46 | 45 |
| WT 5 | 14.29 | 10.33 | 72 | 14.71 | 10.11 | 69 | 6.22 | 5.11 | 82 | 5.48 | 1.24 | 23 | 2.84 | 1.56 | 55 | 2.58 | 0.73 | 28 |
| WT 6 | 15.87 | 13.30 | 84 | 15.83 | 11.77 | 74 | 6.71 | 5.49 | 82 | 6.11 | 1.31 | 21 | 2.77 | 1.27 | 46 | 2.82 | 1.15 | 41 |
| Mean | 16.4 | 13.3 | 81 | 16.0 | 11 | 69 | 6.9 | 5.8 | 85 | 6.5 | 1.4 | 21 | 2.8 | 1.5 | 54 | 2.8 | 1.1 | 38 |
| SD | 1.7 | 2.1 | 6 | 1.0 | 0.7 | 4 | 0.6 | 0.6 | 3 | 0.9 | 0.1 | 1 | 0.2 | 0.2 | 6 | 0.3 | 0.3 | 7 |
| Albinos | ||||||||||||||||||
| ALB 2 | 14.81 | 12.36 | 84 | 15.06 | 10.57 | 70 | 6.35 | 5.72 | 90 | 6.45 | 1.58 | 25 | 3.02 | 1.87 | 62 | 2.95 | 1.31 | 44 |
| ALB 4 | 15.69 | 11.89 | 76 | 15.81 | 11.47 | 73 | 6.80 | 5.80 | 86 | 6.91 | 1.8 | 26 | 2.41 | 1.21 | 50 | 2.84 | 0.87 | 31 |
| ALB 7 | 13.78 | 11.55 | 84 | 13.53 | 10.43 | 77 | 6.18 | 4.49 | 73 | 6.01 | 1.60 | 27 | 2.84 | 1.43 | 50 | 2.66 | 0.98 | 37 |
| Mean | 14.8 | 11.9 | 81 | 14.8 | 10.8 | 73 | 6.4 | 5.3 | 83 | 6.5 | 1.70 | 26 | 2.8 | 1.5 | 54 | 2.8 | 1.1 | 37 |
| SD | 1.0 | 0.4 | 5 | 1.2 | 0.6 | 4 | 0.3 | 0.7 | 9 | 0.5 | 0.1 | 1 | 0.3 | 0.3 | 7 | 0.1 | 0.2 | 7 |
- 1 Total and labelled volumes represent ×10−3 mm3.
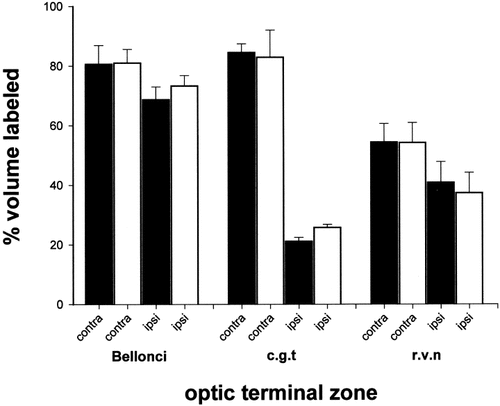
Relative distributions of crossed and uncrossed retinal inputs to the optic centers of the anterior thalamus in wild-type (filled bars) and albino (unfilled bars) Xenopus frogs. The histograms show the mean (+ SD) proportion of each target zone occupied by terminations derived from the contralateral (contra) and ipsilateral (ipsi) eyes.
Distribution of ganglion cells with crossed and uncrossed projections
To assess whether the source of these projections are also similar in normal and albino frogs, ganglion cells in the two eyes were retrogradely labeled from the thalamus. Satisfactory injections, confined to one side of the brain (see Fig. 6), were achieved in five wild-types and five albinos. As summarized in Table 2, there were some differences in their size and location between individuals of each pigmentation phenotype, but with a similar range of placements and structures involved across the two groups. Figure 7 shows fluorescence images of the 4-Di-10-ASP–labeled ganglion cells typically observed, taken from matching ventrotemporal regions of relatively high-density labeling in the contralateral and ipsilateral retinae of an albino frog. Most of the labeled cells had relatively small somata with granular dye confined to their perikarya and primary dendritic processes, and these were clearly far more numerous contralaterally, but some larger ganglion cells were also labeled in all retinae.
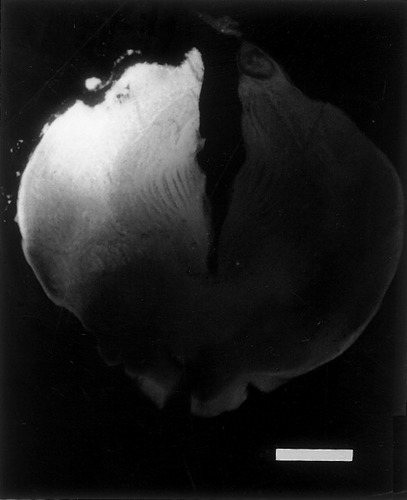
Fluorescent 4-(4-didecylaminostyrl)-N-methylpyridinium iodide (4-10-Di-ASP) tracer deposit in the anterior thalamus (frog ALB 23). This transverse section is taken at a mid-level through neuropil of Bellonci (nB) and corpus geniculatum thalamicum (cgt), which are completely filled with dye. Some diffusion has occurred away from the main injection site, to occupy roughly half of the injected left thalamus. On this basis, it was considered to be a “medium” injection. “Small” injections were more restricted laterally, and “large” injections extended up to the midline ventricle (see Table 2). Scale bar = 1 mm.
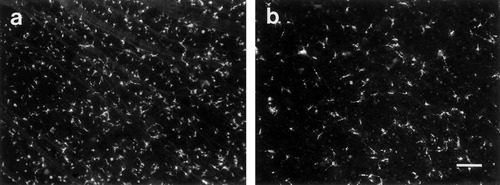
Appearance of 4-(4-didecylaminostyrl)-N-methylpyridinium iodide (4-10-Di-ASP) -labeled ganglion cells in the retina (a) contralateral and (b) ipsilateral to the injected thalamus. The photomicrographs are representative of the higher density cell labeling typically observed in the two retinae and are taken from roughly homotopic mid-temporal locations. Ventral is down in both figures; temporal is to the left in a and is to the right in b. Scale bars = 100 μm in a, 50 μm in b.
The patterns of retinal labeling produced by most of these injections were similar, and indistinguishable between wild-types and albinos. Ganglion cells with crossed projections were usually found in considerable numbers throughout the contralateral retina. Ipsilaterally projecting ganglion cells exhibited a markedly different distribution. In all 10 frogs analyzed, these cells were restricted to a distinct crescent involving only the temporal and ventral retina, with the entire nasal quadrant and regions around the dorsally located optic nerve head devoid of cell labeling. Figure 8 illustrates these features of the uncrossed projections in two normally pigmented and in two albino frogs, with small to medium thalamic injections. As indicated here, and in Figure 9, a continuous line of decussation could be defined in the ipsilateral retina of each frog, by marking the boundary between the labeled ventrotemporal crescent and unlabeled dorsonasal quadrants. An additional feature (Fig. 8) was that the peak densities of ipsilaterally projecting cells were consistently found in a region of the mid-ventrotemporal retina. An area centralis has been identified previously at a similar location in the normal Xenopus retina, based on the peak density of other retinal cells (Schütte and Witkovsky, 1990).
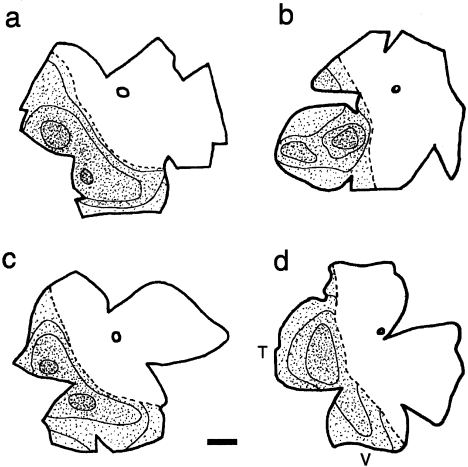
Diagrams of flattened whole-mounted retinae illustrating the distribution of ipsilaterally projecting ganglion cells in two wild-type (a,b) and two albino (c,d) Xenopus frogs. All retinae are oriented similarly: V, ventral; T, temporal, refers to all diagrams. The circle in the nasodorsal quadrant of each retina shows the position of the optic disc. In each diagram, a single dot corresponds to approximately five labeled cells. Isodensity regions, as defined in the text, are outlined. The discontinuous line demarcates the division between the labeled temporoventral crescent and the unlabeled nasodorsal region of each retina. Scale bar = 1 mm in a (applies to a–d).

The nasotemporal division of the Xenopus retina. The photomontage shows a region of temporoventral retina in an albino frog (ALB 23); ventral is down, temporal is to the right. Substantial numbers of ipsilaterally projecting ganglion cells are seen in the right panel of the figure, but these numbers decrease to zero density in the middle panel (i.e., at the line of decussation), and only their labeled axons coursing toward to optic disc are present in the left panel. Scale bar = 50 μm.
Uncrossed retinal ganglion cells: nasotemporal division, numbers, and size
In mammalian albinos, the retinal line of decussation is less well defined and is shifted temporally compared with normally pigmented counterparts. To determine whether these anomalies also occur in albino Xenopus, changes in the density of ipsilaterally projecting ganglion cells were calculated for each retina along a line extending from its central (optic) axis, across the division to the far temporal periphery, and passing through the region of peak labeling density. Figure 10 shows these density changes with retinal eccentricity for each of the wild-type and albino frogs, normalized for between-animal differences in absolute peak label density and retinal distance. In the five wild-type cases, the nasotemporal division was located at between 28% and 40% (mean = 33.6 ± 4.1 SD) of the distance between the optic axis and temporal periphery, and it was located at essentially the same range of eccentricities (28–42%, mean = 34.8 ± 6.1 SD) in the five albinos. Figure 10 also shows that there was a sharp increase in the density of uncrossed cells immediately temporal to the division in all retinae and that the gradient of the density change toward to the area centralis was equivalent in the two pigmentation phenotypes. Thus, the location and definition of the nasotemporal division in the ipsilateral retina was similar in the normal and albino frogs.
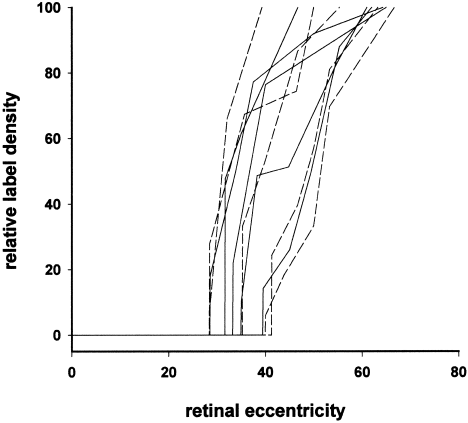
Relative density changes in ipsilaterally projecting ganglion cells across the nasotemporal division in wild-type (continuous lines) and albino (broken lines) Xenopus frogs. The x-axis represents retinal eccentricity, and the y-axis represents cell density, both normalized to subsume between-animal differences in absolute retinal distance and labeled cell number. Zero eccentricity corresponds to the geometric center of the retina (i.e., the optic axis) and 100% to the extreme temporal periphery. The 100% label density corresponds to the area centralis of the mid-temporoventral retina (see Fig. 8).
Quantitative measures of the ventrotemporal retinal area containing uncrossed ganglion cells and estimates of their total number and peak density in the two groups of frog are presented in Table 3. The proportion of retina giving rise to uncrossed projections was similar (range, 36–42%) across individuals, with almost identical means for the wild-types (41.7%) and albinos (40.2%). In mammalian albinos, there are marked reductions in the number of ipsilaterally projecting ganglion cells and this finding is particularly evident at the area centralis. Neither of these anomalies was found in albino Xenopus (Table 3). The average number of uncrossed cells labeled in these frogs (∼2,700) and their peak densities were only slightly less (by approximately 10%) than the equivalent means in the normal animals (e.g., ∼3,000 ipsilaterally projecting cells). These small between-group differences were within the range of the variable error in our cell count procedures and were not statistically significant (P > 0.5, Mann-Whitney U test).
| Animals | Contralateral retina | Ipsilateral retina | |||||
|---|---|---|---|---|---|---|---|
| Area (mm2) | Area labeled (%) | Number of GCs | Area (mm2) | Area labeled (%) | Number of GCs | Peak density (per 0.1 mm2) | |
| Wild-types | |||||||
| WT 10 | 23.6 | 100 | 16,551 | 28.9 | 41.9 | 3,797 | 74 |
| WT 11 | 39.5 | 88.2 | 16,690 | 35.7 | 31.3 | 2,405 | 46 |
| WT 15 | 31.8 | 100 | 17,601 | 32.0 | 44.9 | 3,448 | 54 |
| WT 17 | 36.1 | 100 | 37,947 | 35.9 | 44.8 | 3,151 | 69 |
| WT 19 | 27.5 | 97.5 | 21,356 | 27.6 | 40.4 | 2,474 | 40 |
| Mean | 31.7 | 97.1 | 22,029 | 32.0 | 40.7 | 3,055 | 56.6 |
| SD | 6.4 | 5.1 | 9,110 | 3.8 | 5.6 | 607 | 14.6 |
| Albinos | |||||||
| ALB 16 | 26.1 | 87.8 | 6,993 | 33.0 | 39.7 | 3,261 | 50 |
| ALB 20 | 34.6 | 100 | 26,967 | 33.9 | 41.5 | 3,057 | 56 |
| ALB 22 | 34.1 | 100 | 12,131 | 39.3 | 39.1 | 2,042 | 34 |
| ALB 23 | 30.4 | 100 | 31,392 | 29.5 | 40.7 | 2,260 | 43 |
| ALB 24 | 22.4 | 100 | 30,284 | 22.8 | 40.2 | 2,919 | 70 |
| Mean | 29.5 | 97.6 | 21,553 | 31.7 | 40.2 | 2,708 | 50.6 |
| SD | 5.2 | 5.5 | 11,215 | 6.1 | 0.9 | 528 | 13.6 |
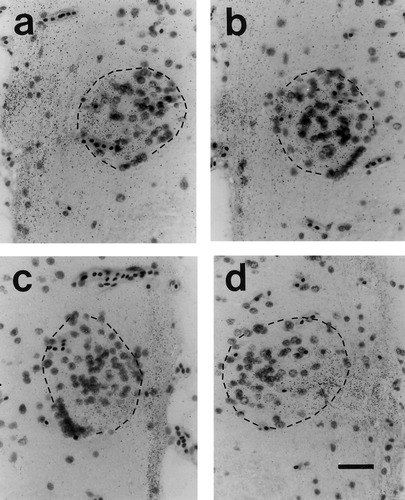
It is possible, however, that the small reductions in the albino frogs could signify a selective deficit in the type(s) of ganglion cell contributing to their uncrossed projections, as has been reported for α cells of the Siamese cat retina (Stone et al., 1978a; Cooper and Pettigrew, 1979; Leventhal, 1982). The retrograde filling achieved with our 4-Di-10-ASP injections did not permit us to identify which of the 12 specific ganglion cell types in the Xenopus retina (Straznicky and Straznicky, 1988) are involved in these projections, as their dendritic morphology was not revealed in sufficient detail. However, as these cells belong to one of three major classes partially identifiable by their (small, medium, or large) soma sizes, measurements of these were undertaken from high magnification (750×) photographs of a selection of well-labeled ipsilaterally projecting ganglion cells, situated between the line of decussation and the area centralis.
As shown in the histograms of Figure 11, two distinct groups of cells, the majority (∼95%) with small (20–85 μm2) soma areas and the rest of medium to large (>120 μm2) areas (Straznicky and Straznicky, 1988), were labeled in the ipsilateral retinae of both the wild-types and albinos. Statistical analyses demonstrated that there were no significant differences in the size distributions of the two samples of ganglion cells, nor between the sizes of the smaller (wild-type mean = 40.9 μm2 ± 14.4 SD, n = 187; albino mean = 43.0 9 μm2 ± 14.4 SD, n = 118: P > 0.2, t test) or larger cell classes (wild-type mean = 150.79 μm2 ± 25.0 SD, n = 10; albino mean = 156.19 μm2 ± 23.9 SD, n = 7: P > 0.6, t test). These data thus provide no evidence for a missing population of ipsilaterally projecting cells in the albino Xenopus retina, further supporting the view that their uncrossed chiasmatic pathways are normal.
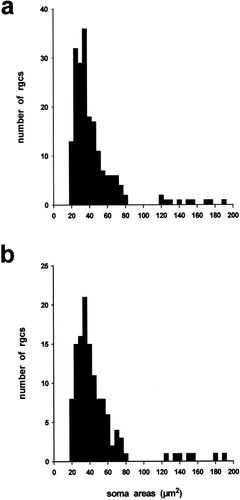
Soma size distributions of ipsilaterally projecting ganglion cells in (a) wild-type and (b) albino Xenopus frogs. The two samples derive from several animals of each phenotype and were taken from similar regions of the temporoventral retina between the line of decussation and the area centralis. Only ganglion cells with dye-labeling that filled the cell body and included the primary dendrites were measured.
DISCUSSION
We compared the retinothalamic projections of wild-type and albino Xenopus with the principal aim of establishing whether the uncrossed components are reduced in frogs lacking RPE melanin. Our results demonstrate that uncrossed projections in normal adults arise from ∼3,000 ganglion cells, equivalent to approximately 5% of the total ganglion cell population (Dunlop and Beazley, 1984), and from a ventrotemporal region comprising ∼40% of the retinal surface. These proportions are quantitatively similar to those, respectively, of normal adult rats (Chan and Guillery, 1993) and cats (Stone et al., 1978b). We also show that uncrossed terminations normally occupy approximately half the combined space available in the nB, cgt, and rvn, albeit with different weightings in each target. Frogs carrying the periodic (ap) tyrosinase gene mutation that we used for comparison had no detectable melanin in their iris, choroid, or skin, and only small amounts of pigment in some cells of the RPE that were probably generated during postembryonic life. But unlike mammals with tyrosinase gene mutations—including some that result in milder RPE pigment deficits than this (e.g., Siamese cats)—we found no evidence that any reductions exist in the uncrossed projections of these albino frogs. Because major deficits in retinal cell numbers commonly found in mammalian albinos (Jeffery, 1997) also do not seem to occur in albino birds or Xenopus (Jeffery and Williams, 1994; Grant et al., 2001), our findings support a general conclusion that RPE melanin deficiency has little influence on the developing retina of nonmammalian vertebrates.
We examined both the origin and termination of the projections to avoid difficulties of interpretation that can arise from examining either one in isolation and to ensure that any abnormality in the albinos was not overlooked. For example, their apparently normal distribution of uncrossed terminations revealed by anterograde labeling could occur despite a major reduction in the number of ipsilaterally projecting ganglion cells, if the remaining cells were larger and had more extensive terminal arbors than those of pigmented frogs. Our complementary data showing that the number and soma sizes of the uncrossed ganglion cell populations are similar in the two phenotypes (Table 3; Fig. 11) allow us to exclude this possibility.
Other difficulties can arise for interpreting retrograde cell labeling when the intended injection site is very small and lies next to fibers of passage that could also take up the tracer. Just such a situation exists in relation to the frog's thalamic visual centers and the adjoining marginal and axial optic tracts, in which the vast majority of fibers are crossed retinal axons bypassing the thalamus en route to the midbrain optic tectum. Involvement of these axons probably accounted for the fact that our larger injections (e.g., in frogs WT17, ALB24) labeled many more ganglion cells in the contralateral retina than the more restricted ones (e.g., in WT15, ALB16). Because such involvement is unavoidable, we cannot provide a reliable estimate of the true number of crossed ganglion cells projecting exclusively to the thalamus. Our main purpose in presenting these data was simply to show that, on average, there was no significant difference between the two groups of frog, suggesting that the mean sizes of their dye injections were also similar. Consequently, our failure to detect a reduction in the number of ipsilaterally projecting cells in the albinos did not arise because we made smaller injections in the wild-types leading us to underestimate their normal numbers.
In any case, there was no relationship between the number of ipsilaterally labeled cells and the size of the dye injections. The probable reason for this is that only two small terminal zones beyond the anterior thalamus—the uncinate and pretectal fields—receive any uncrossed retinal axons in Xenopus (Levine, 1980; Kennard, 1981; Hoskins and Grobstein, 1985a) and many of them have collaterals that also contribute projections to the nB and/or cgt (Steedman et al., 1979). We have examined the ipsilateral projections to these two posterior fields in our autoradiographic material, and they too seem normal in the albinos.
Guillery and Updyke (1976) reported similar findings for albino Ambystoma, although these were only based on anterograde tracing and on two axolotls lacking retinal pigment. Conversely, Dunn-Meynell et al. (1983) have shown, by using anterograde and retrograde methods combined with electrophysiological mapping, that a normally sparse uncrossed retinotectal projection arising from a relatively small number of peripheral ventrotemporal ganglion cells is reduced or absent in albino channel catfish. Whether the affected cells actually misroute their axons at the chiasm (as might be revealed by extra input to the contralateral optic tectum) or whether they belong to a specific, minor cell type whose overall numbers are depleted in albino catfish has not been definitively established. The latter possibility is perhaps the more likely, because the more extensive uncrossed retinal inputs to the normal channel catfish thalamus (Prasada Rao and Sharma, 1982) show no obvious reductions in the albinos (S. Sharma, personal communication). In the remaining discussion, we place our present findings in the context of existing knowledge about the organization and development of uncrossed retinothalamic projections in Xenopus and consider possible reasons why they are not misrouted in albino frogs.
Common features of retinothalamic pathway organization in adult frogs and mammals
One possibility is that the projections have no specific visual function and do not abide by the same rules as those of any mammalian counterparts. Balkema and Dräger (1990) have applied a similar rationale to explain why retinal projections mediating non–visual-image-processing functions (e.g., to the suprachiasmatic nucleus) also seem, uniquely in albino mammals, to be immune to the problem. They pointed out that ganglion cells forming these types of “nonvisual” projection normally show no respect for the retinal line of decussation and disobey other conventions by lacking retinotopic order or interocular segregation in their central target. They argued that it is because these ganglion cells do not respond to the same positional signals as those mediating binocular visual functions that their development is unaffected in albino mammals.
The rvn in the frog is an appendage of the entopeduncular nucleus and its function is obscure (Levine, 1980), so it is possible that it receives this type of rule-defying nonvisual input from the two eyes. The larger nB and cgt, however, are undoubtedly important components of the frog's visual system, as their retinal inputs are topographically organized (Scalia and Fite, 1974, Kennard, 1981) and participate in generating binocular (Keating and Kennard, 1976) and chromatic (Muntz, 1962; Kicliter and Chino, 1976; Fite et al., 1977) responses in postsynaptic neurons of the adjoining LGN. Our [3H]proline data also confirm that their binocular retinal inputs are partially segregated (Levine, 1980; Kennard, 1981; Figs. 2, 3, 5), a feature they share in common with eye-specific projections to different layers of the mammalian LGN.
Even more significantly, it also emerged from our data that the Xenopus retina contains a line of decussation analogous to that of most nonprimate mammals. That is, contralaterally projecting ganglion cells are distributed across the entire retina, but uncrossed projections arise only from a restricted region. Although these basic features have been reported before in normal Xenopus after unilateral HRP injections in the thalamus (Hoskins and Grobstein, 1985a, b), the ventrotemporal division of the ipsilateral retina was not as consistently well demarcated as achieved here (see Figs. 8, 9) and in other recent studies (Marsh-Armstrong et al., 1999; Nakagawa et al., 2000) by using lipophilic fluorescent dyes as the retrograde tracer.
Hoskins and Grobstein (1985a) suggested that the division between ventrotemporal and dorsonasal retina might represent the border between the frogs' binocular and monocular visual fields. But the following argument suggests that it corresponds to the vertical meridian of the entire visual field, just as in mammals. Ophthalmoscopic measurements of the binocular and monocular fields in normal adult Xenopus have shown that they subtend ∼160 degrees and 200 degrees, respectively (Grant and Keating, 1986). This finding means that approximately 80% of the retinal surface views the region of binocular overlap directly above and in front of the animal's head. Our present data show that ipsilaterally projecting ganglion cells occupy exactly half this retinal proportion (Table 3) and, because they are restricted ventrotemporally, are positioned to view the furthest region of binocular overlap, which could correspond to the opposite hemifield. Direct electrophysiological recordings from uncrossed retinal terminations in nB and cgt support this contention (S. Grant and C. Kennard, personal communication). Because these projections to the frog thalamus follow the same principle of partial decussation as those mediating binocular vision in mammals, their resistance to misrouting in albino frogs must have some other developmental explanation.
Development of uncrossed retinothalamic projections: frogs vs. mammals
The normal development of uncrossed retinothalamic projections in Xenopus differs from those to the mammalian LGN in two obvious ways. First, the projections do not appear until quite late in development, at mid-larval stages 53/54, when crossed inputs to the thalamus and midbrain have been established for approximately a month (Kennard, 1981; Hoskins and Grobstein, 1985b). Second, their development is prolonged (Hoskins and Grobstein, 1985b), this being associated with progressive enlargement of the binocular field and continued ventrotemporal retinal neurogenesis during metamorphic and postmetamorphic life (Grant and Keating, 1986). In mammals, by contrast, the first ganglion cells that will form permanent uncrossed projections are born relatively early in the development of the central retina and in close spatial and temporal proximity to the first-born cells that will form crossed projections from the other side of the nasotemporal divide (Dräger, 1985a; Reese et al., 1992; Baker and Reese, 1993).
This peculiarity of retinal development in mammals, compared with other vertebrates, suggests a need for especially tight control of the positional signals that specify the correct chiasmatic pathway choices of the two early born ganglion cell populations. Birthdating studies have shown that key spatial and temporal aspects of ganglion cell genesis are disrupted in the albino retina (Ilia and Jeffery, 1996, 1999; Rachel et al., 2002). This disruption could cause ganglion cells on the temporal side of the normal division to miss the signal(s) specifying an uncrossed identity, resulting in misrouting of their axons at the chiasm and a peripheral shift in the line of decussation. Later in development, further uncrossed cells are normally added to the temporal retinal periphery but, interestingly, the equivalent later born cells in albinos are less susceptible to misrouting, even in rodents (Bunt et al., 1983; Dräger, 1985a; Balkema and Dräger, 1990) and carnivores (Leventhal, 1982; Morgan et al., 1987; Reese et al., 1992; Baker and Reese, 1993) in which crossed ganglion cells are added simultaneously to the same region. These findings clearly suggest that the presence of RPE melanin is critical for the mechanism(s) determining uncrossed pathway choice of early born ganglion cells of the mammalian retina, but not for those appearing later in development. If the same were to apply to the late developing uncrossed cells in Xenopus, this could partially explain why they do not misproject in the albino.
Periodic albino Xenopus phenotype
Two alternative explanations merit consideration. In the albino frogs examined, the few RPE cells containing any melanin were likely to have been generated at the same larval stages as the initial ipsilaterally projecting ganglion cells. This is a suspicious coincidence and might suggest that sufficient pigment was available to support adequate specification of their chiasmatic pathway choice. We can reject this possibility on the following grounds. Tay and Straznicky (1977) and Kennard (1981) have examined the ipsilateral projections of “compound eyes” consisting of two temporal (TT) or two nasal (NN) half-retinae, created by adding donor to host tissues in stage 32 embryos. TT eyes were later found to project supranormally to the ipsilateral thalamus, and Kennard (1981) showed by focal lesioning methods that this was due to an additional contribution from the (nasally positioned) half-temporal retina from the donor embryo. NN combinations, by contrast, formed no or only weak uncrossed projections, including the donor hemiretina placed in a temporal position in the host. In other words, the ventrotemporal retina in Xenopus is specified to form uncrossed projections before embryonic stage 32 and long before the participating ganglion cells are born. More recent experiments by Nakagawa et al. (2000) have shown that specification involves uncrossed cells acquiring high levels of EphB receptors and that this, in turn, causes their axons to avoid the chiasmatic midline when cells there begin expressing the “repulsive” ligand, ephrin-B, in mid-larval life. The RPE in albino Xenopus, however, never expresses any pigment until stage 40 (Hoperskaya, 1975) at least a day after specification normally occurs and too late to influence these past events.
When retinal axons enter the developing optic nerve in normal Xenopus they encounter a group of melanin-containing astrocytes at a location where they radically change their trajectory (Silver and Sapiro, 1981; Taylor, 1993). A similar group of optic stalk cells transiently expresses pigment in some wild-type mammals but not in their albino counterparts (Silver and Sapiro, 1981; Webster et al., 1988). These authors suggested that the pigmented cells may act as guideposts, selectively directing axons from temporal retina toward a position in the prechiasmatic nerve that disposes them to take an uncrossed course, with consequent misrouting in animals in which they are absent. An unusual feature of albino Xenopus is that the pigmented astrocytes are actually present and, as in normal frogs, continue to express melanin into adulthood. There are, however, compelling grounds for dismissing this seemingly obvious reason for their normal uncrossed pathways. First, Taylor (1993) has demonstrated that the change in trajectory occurring near the pigmented cells in both normal and albino Xenopus does not separate axons according to their future chiasmatic route, but generates an age-related order that facilitates retinotopic map formation. Second, melanin is absent in some pigmented strains of rat in which the uncrossed pathways develop normally (Horsburgh and Sefton, 1986; Collelo and Jeffery, 1991), so there is no consistent relationship between optic stalk melanin and chiasmatic routing, even in mammals.
CONCLUSIONS
In summary, the major chiasmatic misrouting common to hypopigmented mammals does not generalize to albino Xenopus, despite the adult wild-type retina possessing a well-defined ventrotemporal division and substantial uncrossed projections. The reasons for this fundamental difference remain unknown but may be related to a peculiar requirement in mammals to generate simultaneous projections from both sides of the nasotemporal division early in development. Mammals, thus, may have evolved unique mechanisms, perhaps involving melanin-dependent RPE gap junctional signaling (Dräger, 1985b), to meet this special need. Frogs and mammals may, nonetheless, share conserved mechanisms of uncrossed ganglion cell specification. Nakagawa et al. (2000) have shown that cells of the mammalian chiasm also express ephrin-B early in development, suggesting that this molecule may be a common repulsive signal for EphB-bearing uncrossed axons in normal (Godement et al., 1990, 1994; Wizenmann et al., 1993; Marcus et al., 1995; Wang et al., 1995) and albino mammals (Marcus et al., 1996), as well as in frogs. It would be interesting to compare the distributions of EphB-positive ganglion cells in the developing retina of normal vs. albino frogs and mammals, to further explore their relations with RPE melanin expression and chiasmatic pathway choice.
Acknowledgements
We thank Gary Baker and Glen Jeffery for comments on the manuscript. We also thank Eugenia Diaz, Glen Jeffery, George Mentis, Linda Moran, and Roberto Navarrete for their help and advice.




