Mechanism of Ozone Oxidation of Polycyclic Aromatic Hydrocarbons During the Reduction of Coking Wastewater Sludge
Abstract
Activated coking wastewater sludge is a significant problem, due to the content of adsorbed hydrophobic organic micro-pollutants which are adsorbed. Here we discuss an O3 fluidized bed reactor (FBR) process able to stabilize and reduce the coking sludge, and also removing 16 target polycyclic aromatic hydrocarbons (PAHs) adsorbed onto the sludge. The degradation efficiencies and influential factors affecting the sludge ozonation, such as the concentration of O3 and of H2O2, UV irradiation, and the initial pH were investigated. The results indicate that the target PAHs present in the activated coking sludge, especially those with high molecular weight can be effectively removed by O3 FBR. However, the concentration of O3 plays an important role in the degradation of the target PAHs because a low concentration can generate an increase in the concentrations of PAHs in the liquid phase of the activated coking sludge. Due to the synergistic effect (S > 0), increasing the concentration of H2O2 and increasing the pH value, can improve the removal of most of the target PAHs. The degradation kinetics of the target PAHs are assigned to a pseudo first-order model. In this process, the solubilization rate of the activated coking sludge reached about 68.2% and the target PAHs adsorbed on it were removed by >95% at 500 mg O3 g−1 SS.
Abbreviations
-
- Ace
-
- acenaphthene
-
- Acy
-
- acenaphthylene
-
- Ant
-
- anthracene
-
- BaA
-
- benzo[a]anthracene
-
- BaP
-
- benzo[a]pyrene
-
- BbF
-
- benzo[b]fluoranthene
-
- BgP
-
- benzo[g,h,i]perylene
-
- BkF
-
- benzo[k]fluoranthene
-
- Chr
-
- chrysene
-
- COD
-
- chemical oxygen demand
-
- DBA
-
- dibenzo[a,h]anthracene
-
- FBR
-
- fluidized bed reactor; HMW, high molecular weight
-
- Fle
-
- fluorine
-
- Flu
-
- fluoranthene
-
- IndP
-
- indeno[1,2,3-cd]pyrene
-
- Naph
-
- naphthalene
-
- PAH
-
- polycyclic aromatic hydrocarbon
-
- Phen
-
- phenahthrene
-
- Pyr
-
- pyrene
-
- TOC
-
- total organic carbon
-
- TSS
-
- total suspended solids
-
- VSS
-
- volatile suspended solids
-
- WWTP
-
- wastewater treatment plant
1 Introduction
The activated sludge process is widely applied to coking wastewater. However, the aerobic biological treatments involve the production of large amounts of activated sludge which become a serious issue for pollution control 1. Coking wastewater contains considerable amounts of phenols, polycyclic nitrogen-containing aromatics, oxygen- and sulfur- containing heterocyclic, and acyclic compounds 2. Due to their hydrophobic and lipophilic nature, polycyclic aromatic hydrocarbons (PAHs) tend to be adsorbed onto the sludge and be accumulated during wastewater treatment processes. Hua et al. showed that all 16 PAHs targeted by the United States Environmental Protection Agency (US EPA) were present in the activated sludge from 12 municipal wastewater treatment plants from the Zhejiang province of China with a total concentration ranging from 33.73 to 82.58 mg kg−1 based on dry weight 3. Some wastewater treatment plants in France, Spain, United Kingdom, and Switzerland have also been shown to have mean concentrations of these target PAHs in the range of 1–2000 mg kg−1 based on dry weight 4-7. In addition, the concentration of the 16 PAHs in the samples of coke plant wastewater from the Zdzieszowice Coke Plant, Poland, varied between 255.05 and 133.91 μg L−1. About 71–84% of the PAHs were adsorbed on the surface of suspended solids 8. However, the target PAHs concentrations in coking wastewater (5466.3 ± 86.3 μg L−1), as well as the concentrations of selected PAHs in coking sludge samples (4068.6 ± 133.4 to 4861.4 ± 353.7 mg kg−1) from a coking production plant in the Guangdong province of China were considerably higher 9, 10. This province has a higher incidence of cancer which may be linked to the high PAH levels emphasizing the need to reduce PAH concentrations in the coking sludge.
The conventional methods for sludge treatment and disposal (e.g., landfill and incineration) 11 are confronting severe challenges due to more stringent environmental regulations. Various novel reduction technologies to treat excess sludge have been developed, such as thermal, ultrasonic, mechanical, alkaline, or oxidative treatments 12-15, along with biological treatment processes. Among these methods, sludge ozonation is considered to be one of the most effective techniques, having the highest cell disintegration capability. It has been successfully applied to reduce sludge in several European countries, as well as in Japan 16, 17. Biodegradation of low molecular weight PAHs by both bacteria and fungi has been well documented, but is not applicable to high molecular weight (HMW) PAHs 18. The refractory nature of HMW PAHs was partly attributed to their low solubility/bioavailability, together with their low degradation rates possibly limited by dissolution or desorption 19. Ozone can offer a rapid and aggressive alternative that is not sensitive to the type and concentration of the contaminant 20. PAH degradation by anaerobic digestion could be improved by ozone pre-treatment 21. However, this method presents some disadvantages. For example, some refractory persistent organic contaminants originally adsorbed onto the sludge may get released into the wastewater during sludge ozonation, leading to the reduction of the quality of the effluent. Two questions thus arise regarding the practical application of sludge ozonation. Firstly, will sludge solubilization lead to an increase in the concentrations of PAHs in the wastewater? Secondly, can ozonation effectively degrade the PAHs that were originally adsorbed by the sludge and dissolve them in the wastewater? There is currently a lack of research into these issues and thus the current study addresses these gaps in the literature.
In addition, there is a still a need to develop more efficient techniques to enhance ozone mass transfer, to treat waste ozone gas, and to reduce the cost of sludge ozonation. Internal loop fluidized bed reactors (FBR) are a new type of multiphase reactor, which have a number of advantages, These include, having a simple structure, a good mixing, and mass transfer effects, being easy to operate and maintain, and having low energy requirements per unit of volume 22. Such reactors have been widely used in many fields, such as in the petro-chemical industry, as well as for biological fermentation and wastewater treatment 23. A previous study by Zhang 24 presented the effect of the cross-shape baffle on the hydrodynamic properties in a bench-scale reactor, as well as the enhancement of local or global gas holdup, and the reduction of turbulent kinetic energy. Although ozonation of sewage sludge has been extensively investigated, the application of an ozone FBR to reduce PAHs in coking wastewater sludge has not been widely documented in the literature.
The main objectives of this study are to: (1) investigate the degradation efficiency of the target PAHs during the sludge ozonation by an ozone FBR; and (2) examine the effects of H2O2, UV irradiation, and pH value of the sludge during the degradation of PAHs. The results of this study will help to optimize the coking wastewater sludge ozonation process in an O3 FBR to reach efficient sludge reduction and PAHs degradation.
2 Materials and methods
2.1 Target PAHs and sludge source
Sixteen PAHs were selected as the representative PAHs whose structures, partitioning coefficient logKow and carbon normalized sorption coefficient Koc are shown in Supporting Information Tab. S1. The deuterated surrogates were naphthene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12. Hexamethylbenzene was used as an internal standard for gas chromatography (GC) analyses and was obtained from Sigma–Aldrich (Gillingham, Dorset, VT). All solvents used for sample preparation and analysis (dichloromethane, hexane, acetone, and methanol) were HPLC grade from Merck (Darmstad, Germany). Deionized water was produced by a Milli-Q system (Millipore, Bedford, MA). The activated sludge was collected from the Songshan coking wastewater treatment plant (WWTP) located in Shaoguan, Guangdong province in China, having an average treatment capacity of 2000 m3 · day−1. The ammonia of effluent and cleaning wastewater were collected from the influent of the coking WWTP. The primary treatments included a flotation-degreasing tank and an equalization basin. The particles and tar separated in the flotation-degreasing tank were oxidized by the Fenton method, while the primary effluent was directed to the biological system. An anoxic–oxic–hydrolytic–oxic system coupled with a biological fluidized bed was applied at the biological stage, and pressurized air was injected into the biological tanks. The biological sludge from various tanks of the biological stage were mixed with a thickener, and then pumped to the dewatering unit. The concentrations of the total suspended solids (TSS) and volatile suspended solids (VSS) were about 5.7 and 4.4 g L−1, respectively, and the pH value was about 7.3. The mean concentrations of regularly measured parameters (e.g., chemical oxygen demand (COD), volatile phenols, NH4+-N and CN−) are shown in Supporting Information Tab. S2, with the average removal efficiencies ranging from 96 to 99%.
2.2 O3 FBR
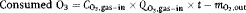 (1)
(1)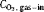 represents the gaseous concentration in of O3 (mg L−1) at the inlet;
represents the gaseous concentration in of O3 (mg L−1) at the inlet; 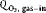 represents the flow rate of O3 gas; t is the duration (min) of the sludge ozonation; and
represents the flow rate of O3 gas; t is the duration (min) of the sludge ozonation; and  is the total mass (mg) of O3 in the off-gas. The amount of O3 consumed could be readily controlled by adjusting either the ozonation duration or the electrical intensity of the O3 generator.
is the total mass (mg) of O3 in the off-gas. The amount of O3 consumed could be readily controlled by adjusting either the ozonation duration or the electrical intensity of the O3 generator.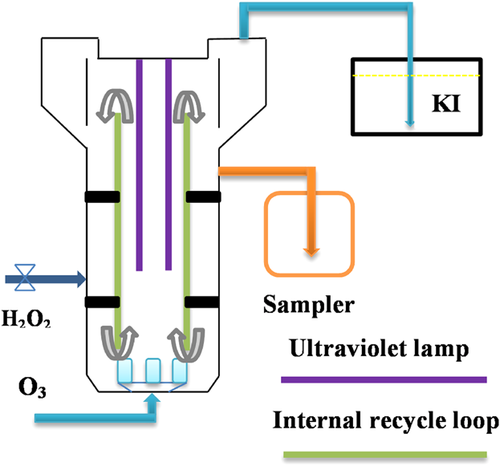
2.3 Experimental procedure
Before each experiment, pure oxygen was injected into the reactor to stir the coking sludge. The ozone generator was switched on when the power and the inlet flow were stable. In all oxidation experiments, the initial concentrations of the target PAHs in the coking sludge have been determined as shown in Tab. 1. The reaction temperature was fixed at about 25°C. Samples were collected in an amber glass bottle and analyzed in duplicate for each sampling time.
| Naph | Acy | Ace | Fle | Phen | Ant | Flu | Pyr | |
|---|---|---|---|---|---|---|---|---|
| Solution (ng L−1) | 0.54 ± 0.11 | 0.81 ± 0.12 | 0.95 ± 0.15 | 0.86 ± 0.14 | 1.38 ± 0.13 | 1.45 ± 0.12 | 3.76 ± 0.16 | 2.87 ± 0.13 |
| Sludge (ng g−1) | 5.61 ± 0.82 | 30.8 ± 3.81 | 7.3 ± 0.52 | 4.9 ± 0.43 | 23.0 ± 2.62 | 10.9 ± 0.61 | 732 ± 40.4 | 607 ± 28.4 |
| BaA | Chr | BkF | BbF | BaP | DBA | IndP | BgP | |
| Solution (ng L−1) | 2.1 ± 0.14 | 2.24 ± 0.15 | 1.93 ± 0.16 | 1.11 ± 0.12 | 2.3 ± 0.13 | 1.5 ± 0.16 | 1.31 ± 0.18 | 1.7 ± 0.15 |
| Sludge (ng g−1) | 645 ± 32.7 | 732 ± 39.5 | 860 ± 49.3 | 1050 ± 70.1 | 304 ± 79.5 | 173 ± 29.3 | 780 ± 30.1 | 689 ± 43.7 |
Five experiments were performed under different ozonation conditions. The aim of experiment 1 was to determine the optimal range of the O3 dose by examining the solubilization characteristics of the coking sludge. The objective of the experiment 2 was to investigate the degradation efficiency of the target PAHs in coking sludge at various O3 doses. In experiment 3, the effect of UV irradiation on PAHs removal during coking sludge ozonation was assessed with a specific ozone dose, and in the presence and the absence of a UV irradiation. In experiment 4, the effect of adding H2O2 on PAHs removal during the sludge ozonation was determined by changing the dosing mode of H2O2 (i.e., batch or continuous mode) and varying the molar ratio of H2O2 to O3 (nH2O2/nO3). In the continuous mode, a total of 10 mL H2O2 solution was evenly pumped in to the reactor throughout the sludge ozonation process. In the batch mode the same amount of H2O2 solution was added into the reactor at the beginning of the reaction. In both modes, the O3 dose was set at 400 mg O3 g−1 SS with nH2O2/nO3, 1:1. To examine the effect of the molar ratio of H2O2 to O3, two ratios (1.5:1 and 2:1) were also used in the batch mode. The purpose of experiment 5 was to investigate the effect of the sludge pH on the PAHs removal by adjusting the pH to 5 with 40% H2SO4 v/v or to 9 with 40% NaOH w/w. In this experiment, the dose was maintained at about 400 mg O3 g−1 SS. All experiments were conducted in duplicate at ambient temperature (23 ± 2°C).
2.4 Analysis
PAHs were analyzed by GC/MS (Agilent, 7890A-5973C) in the selected ion mode with a 30 m × 0.25 mm id × 0.25 μm film thickness HP-5MS fused silica capillary column. The GC/MS conditions for sample analysis were as follows: the injection port, interface line and ion source temperature were maintained at 280, 290, and 250°C, respectively. The column temperature was programmed from 60 to 310°C at 5°C · min−1 and held for 10 min. Helium was the carrier gas at a flow rate of 1.2 mL min−1 with a linear velocity of 42.4 cm s−1. The mass spectrometer was operated in the electron impact ionization mode (70 eV). The sample (1 μL) was injected with a split ratio of 10:1.
Because of the adsorption of the PAHs, the samples must first undergo a preparation before further analysis. The sludge from O3 FBR in each series of experiment was taken for analysis. The samples were filtered through a glass filter to separate the liquid from the solid phases. The solid phase was freeze dried and kept at − 20°C before analysis. The solid sample adsorbed onto the glass filter was spiked with 20 μL of surrogate standards (80 μg mL−1) and extracted with a Soxhlet apparatus with 200 mL of dichloromethane for 48 h in a water bath maintained at 46°C. Based on the contents and concentrations of the organic compounds, 1000 mL of wastewater was extracted with C18 cartridges, to which 20 μL of the surrogate standards (80 μg mL−1) were added to correct the losses along the extraction process and provide the efficiency of the extraction. Activated copper was then added to the flask to remove sulfur from the extract.
The extract of the aqueous and solid phase were separately loaded onto a 1:2 alumina/silica gel glass column with 1 g anhydrous sodium sulfate overlaying the silica gel for clean-up and fractionation. A volume of 15 mL hexane was first applied to remove aliphatic hydrocarbons. The eluents containing PAHs were then collected by eluting 70 mL dichloromethane/hexane (3:7, v/v), and were concentrated to 0.5 mL under a gentle stream of pure N2. Internal standards (5 μL, 100 μg mL−1) were added to the sample prior to the GC/MS analysis.
Quantification was determined using a seven-point calibration curve established from a hexane-based internal standard for each individual PAH. The R2-values of PAH calibration curves were all >0.99. The detection limits of the method ranged from 0.06 to 16.56 mg kg−1. The average recoveries for all the sludge samples were 60.1 ± 5.6% for naphthalene-d8, 74.5 ± 10.1% for acenaphthene-d10, 88.7 ± 8.8% for phenanthrene-d10, 98.3 ± 8.5% for chrysene-d12, and 96.4 ± 9.6% for perylene-d12.
CODCr, TOC, and pH values were analyzed according to the Chinese State Environment Protection Agency (SEPA) Standard Methods (Chinese SEPA, 2002). TOC was measured with a TOC analyzer (TOC-VCPH, Shimazdzu, Japan) after filtration through a 0.45 μm membrane. The pH value was measured with a pH meter (pHS-3C, P. R. China).
2.5 Date processing
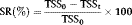 (2)
(2)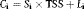 (3)
(3)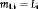 (4)
(4)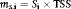 (5)
(5)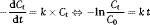 (6)
(6)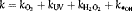 (7)
(7)3 Results and discussion
3.1 Sludge solubilization characteristics by O3 FBR
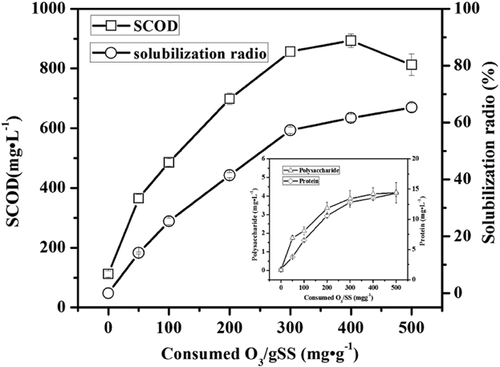
Figure 2 shows the effect of the O3 concentration on the solubilization of the coking sludge. As the concentration of O3 increased, the solubilization rate raised almost linearly until 300 mg O3 g−1 SS, after which point it reduced. At 500 mg O3 g−1 SS, the solubilization rate reached the highest value of 68.2%. Previous studies showed that O3 concentrations as low as 50 mg O3 g−1 SS effectively reduced the excess of sludge production 25. As the concentrations of the PAHs and other organic substances in the coking activated sludge in this study were much higher compared to published studies, higher O3 concentrations were needed to destroy the activated sludge 26. The solubilization of the activated sludge was the key process of the sludge reduction. Figure 2 illustrates that the concentration of soluble COD increased dramatically to 879 mg mL−1 at 400 mg O3 g−1 SS compared to the initial value. The amount of proteins and polysaccharides that were measured in the sample also indicated that the sludge and microbial cells were destroyed. A distinct increase of the soluble COD concentration can be observed (Fig. 2) because O3 disrupts the membranes of the microbial cells leading to the release of intracellular substances into the liquid phase. Figure 2 also shows that the total COD in the sludge was raised to a maximum at 400 mg O3 g−1 SS, and then decreased considerably fast as the concentration of O3 increased. At 500 mg O3 g−1 SS, the total COD concentration was reduced by about 100 mg L−1, implying segmental mineralization of organic substances by O3 oxidation. In the view of large scale applications, the O3 concentration should be optimized for economic reasons 25, 27. Therefore, an O3 concentration ranging from 0 to 500 mg O3 g−1 SS was tested, with a sludge solubilization rate being close to 68.2% at 500 mg O3 g−1 SS.
3.2 Effects of the ozone concentration on the reduction of the coking sludge
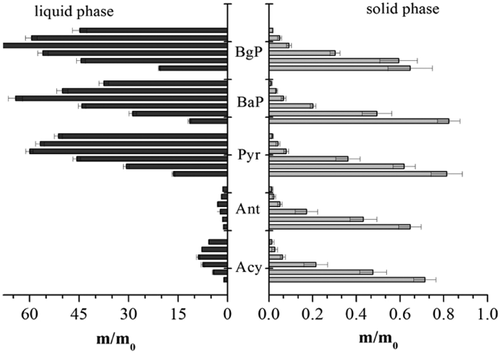
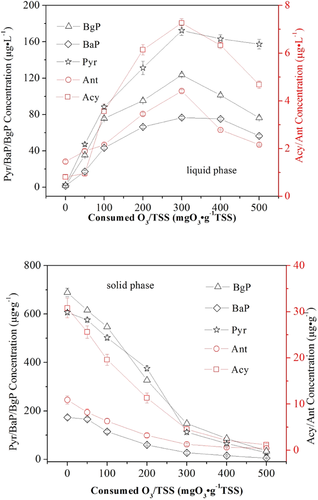
The changes in the amounts of PAHs distributed in the liquid and solid phases of the activated sludge at different O3 concentrations are shown in Fig. 3 and Supporting Information Fig. S1. The results indicated that the removal rates of the target PAHs were the same in the different sludge phases, even at a concentration of 500 mg O3 g−1 SS, and that the removal efficiencies were >95%. Furthermore, the dissolution rate of fluorine (Fluo), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), dibenzo[a,h]anthracene (DBA), indeno[1,2,3-cd]pyrene (Indp), pyrene (Pyr), benzo[g,h,i]perylene (BaP) and BgP were much faster than those of naphthalene (Naph), acenaphthene (Ace), fluoranthene (Flu), phenahthrene (Phe), acenaphthylene (Acy), and anthracene (Ant) in the liquid phases until the O3 concentration reached 300 mg O3 g−1 SS. After this point, the target PAHs were almost simultaneously degraded as the O3 concentration increased both in the liquid and solid phases of the coking activated sludge. This phenomenon implies that the PAHs in the coking activated sludge were probably degraded through three pathways. Firstly, the PAHs were released from the solid phase to the liquid phase during the process of membranolysis; secondly, the dissolved O3 oxidized the PAHs distributed in the liquid phase; lastly the PAHs adsorbed on the solid phase were directly oxidized by the dissolved O3. Moreover, the chemical structures of the organic substances were significantly altered by O3 compared to their parent compounds, leading to an increase of the biodegradability of the solution 28. The results shown in Supporting Information Fig. S2 also indicate that a significant sludge reduction appeared in the O3 FBR during the O3 oxidation. The TSS concentration of the coking activated sludge quickly dropped as the concentration of the dissolved O3 increased. At the same time, the TOC concentration of the liquid increased rapidly as the O3 concentration increased and then decreased. This observation confirmed that the organic substances released from the coking sludge could be partially mineralized if sufficient O3 was supplied to the system. Nevertheless, the organic substances in the liquid phase could not be removed thoroughly according to the residual TOC because of their enhanced hydrophilicity after the oxidation process 29.
3.3 Degradation kinetics of PAHs
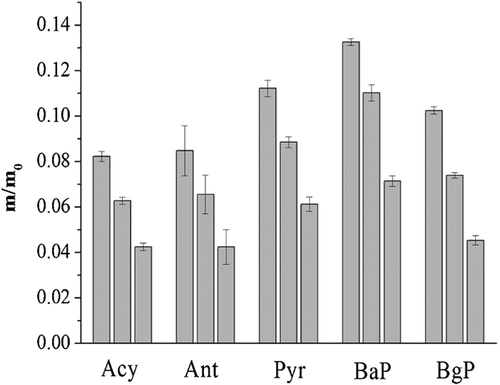
The changes in the concentration of the target PAHs in the solid and liquid phases of the coking activated sludge during sludge reduction are shown in Fig. 4 and Supporting Information Fig. S3. The target PAHs Acy, Ant, Pyr, BaP, and BgP exhibited the same removal trends in the liquid phase. The concentrations increased gradually as the O3 concentration increased, until a concentration of 300 mg O3 g−1 SS, and then the concentration gradually decreased. The initial increase and the gradual decrease in the concentrations of the target PAHs demonstrate that the degradation by O3 in the liquid phase was lagged behind the release from the coking sludge during the sludge reduction. All target PAHs concentrations decreased as the O3 concentration increased in the solid phase. The dissolved O3 may alter the chemical structures of the activated coking sludge causing the release of the sludge-bound PAHs, and the direct reaction with them. The removal efficiencies of HMW PAHs were also higher in the solid phase than the liquid phase one. The adsorption process of the target PAHs in the coking activated sludge can undergo surface adsorption or partition 30. Obviously, in the first stage, the PAHs adsorbed on the sludge granules surfaces were released into the liquid phase during the initial stage of ozonation because of the solubilization of extracellular polymeric substance. And then, the dissolved PAHs in the liquid phase degraded together with the sludge reduction 28. This infers that a low O3 concentration may lead to a significant increase in the concentrations of PAHs in the wastewater. Therefore, the practical O3 concentration should be carefully controlled during sludge ozonation in the O3 FBR.
The degradation rates of PAHs during oxidation were affected by the concentrations of O3 and PAHs, as well as the reaction rate constants. In this work, O3 was continuously supplied to the FBR and its aqueous concentration remained almost at a steady-state (about 0.13 mg L−1). In order to simplify the kinetic model, the degradations of PAHs in both the solid and liquid phases of activated coking sludge were taken into consideration, thus the apparent rate constant for the reaction of an individual PAH with O3 in activated coking sludge (ks) could be determined. Therefore, the reactions of O3 with the target PAHs in the sludge could be approximately expressed by the pseudo-first-order kinetics. The plot of ln(Ci/Ci,0) versus t yielded a linear curve with the slope equaling ks’ under the experimental conditions of pH 7.3, and at ambient temperature of 23 ± 2°C (Supporting Information Fig. S4). The values of ks were calculated from ks’ and are summarized in Tab. 2. It is noticed that the apparent reaction rates in the coking sludge ranged from 1.91 × 103 to 2.62 × 103 M−1 s−1. Among the target PAHs, the reaction rates of the HMW PAHs in the coking sludge were higher than those of low molecular weight PAHs. A comparison of the logKow values (ranging from 3.37 to 6.75) shows that HMW PAHs are more easily absorbed on the coking sludge granules, and that the initial amount of HMW PAHs absorbed on the solid phase reached a maximum of 96% (Tab. 1). The adsorption or desorption of the target PAHs on the activated coking sludge greatly restricted their degradation during ozonation. In addition, the efficiency of the PAH degradation was highly dependent on the initial concentrations of the PAHs in the coking sludge, with greater degradation observed in the sludge that contained high initial concentrations of these contaminants. A number of experimental studies have explored heterogeneous reactions of PAHs with ozone 31, 32. The results indicate that these reactions proceed via a Langmuir–Hinshelwood mechanism that involves two processes: (1) equilibrium partitioning of the gas phase oxidants between the gas phase and the surface; and (2) surface reactions between the ozone and the adsorbed molecules 33. Therefore, ozone may initially be physisorbed to the surface of the sludge, after which it is transformed to a reactive intermediate prior to reacting with the PAHs. Huber et al. 34 also showed that the adsorbed micro-pollutants on sludge granules would not be oxidized efficiently by O3 because the transfer of gaseous O3 from gas bubbles to the liquid phase is much faster than that of aqueous O3 from the liquid phase to the surface of the sludge.
| PAHs | ks′ (s−1) | ks (×103 M−1 s−1) | R2 |
|---|---|---|---|
| Naph | 0.0071 | 2.62 | 0.99 |
| Ace | 0.0052 | 1.91 | 0.98 |
| Acy | 0.0067 | 2.48 | 0.99 |
| Flu | 0.0054 | 1.98 | 0.97 |
| Phe | 0.0057 | 2.10 | 0.97 |
| Ant | 0.0062 | 2.31 | 0.98 |
| Fluo | 0.0060 | 2.23 | 0.98 |
| Pyr | 0.0061 | 2.27 | 0.97 |
| Chr | 0.0069 | 2.56 | 0.98 |
| BaA | 0.0064 | 2.38 | 0.98 |
| BkF | 0.0068 | 2.51 | 0.99 |
| BbF | 0.0068 | 2.53 | 0.99 |
| BaP | 0.0055 | 2.04 | 0.98 |
| DBA | 0.0059 | 2.18 | 0.99 |
| Indp | 0.0064 | 2.36 | 0.99 |
| BgP | 0.0068 | 2.52 | 0.99 |
3.4 Effect of H2O2 addition and ultraviolet (UV) irradiation
In the O3 FBR, direct O3 oxidation and indirect oxidation by  formed from the reaction of O3 could lead to the target PAHs degradation. During advanced oxidation processes (AOPs), the combination of O3 with H2O2 and UV has been extensively applied to enhance
formed from the reaction of O3 could lead to the target PAHs degradation. During advanced oxidation processes (AOPs), the combination of O3 with H2O2 and UV has been extensively applied to enhance  generation due to the cumulative or synergistic effects 35, 36. In this work, H2O2 was thus added to evaluate its contribution to the PAH degradation during the sludge ozonation by varying the molar ratio of H2O2 to O3
generation due to the cumulative or synergistic effects 35, 36. In this work, H2O2 was thus added to evaluate its contribution to the PAH degradation during the sludge ozonation by varying the molar ratio of H2O2 to O3 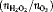 and the H2O2 dosing mode.
and the H2O2 dosing mode.
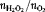 increased from 0.5 to 1.0, the removal of the target PAHs both in the liquid and sludge phases were enhanced. However, the removal efficiency decreased as the H2O2 concentration increased. It can be noted that the concentration of the target PAHs almost remained constant as the
increased from 0.5 to 1.0, the removal of the target PAHs both in the liquid and sludge phases were enhanced. However, the removal efficiency decreased as the H2O2 concentration increased. It can be noted that the concentration of the target PAHs almost remained constant as the 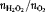 increased from 1 to 2. There are two explanations for this trend. Firstly, many organic and inorganic substances could compete for
increased from 1 to 2. There are two explanations for this trend. Firstly, many organic and inorganic substances could compete for  as scavengers, such as
as scavengers, such as 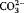 ,
, 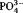 , and CH3COOH in the coking activated sludge 37. Secondly, the decomposition of aqueous O3 could be accelerated by H2O2, and thus weakened the direct oxidation of PAHs by molecular O3. Therefore, an equilibrium between the strengthened indirect oxidation via
, and CH3COOH in the coking activated sludge 37. Secondly, the decomposition of aqueous O3 could be accelerated by H2O2, and thus weakened the direct oxidation of PAHs by molecular O3. Therefore, an equilibrium between the strengthened indirect oxidation via  and the weakened direct oxidation via molecular O3 could be established in the combination of O3 with H2O2. Coupling H2O2 to ozonation is a way to improve the efficiency of the process due to cumulative or synergistic effects 38. The pseudo first-order rate constants of the ozonation
and the weakened direct oxidation via molecular O3 could be established in the combination of O3 with H2O2. Coupling H2O2 to ozonation is a way to improve the efficiency of the process due to cumulative or synergistic effects 38. The pseudo first-order rate constants of the ozonation 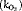 , photocatalysis
, photocatalysis 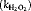 and the O3/H2O2 oxidation
and the O3/H2O2 oxidation 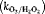 were calculated and the degree of synergy, S, was determined according to Eq. 8.
were calculated and the degree of synergy, S, was determined according to Eq. 8.
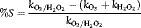 (8)
(8)
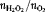 ratio: 0, 0.5, 1.0, and 2.0 (from left to right). The O3 dose was about 400 mg O3 g−1 SS. The decay curves for other target PAHs are similar (Supporting Information Fig. S5).
ratio: 0, 0.5, 1.0, and 2.0 (from left to right). The O3 dose was about 400 mg O3 g−1 SS. The decay curves for other target PAHs are similar (Supporting Information Fig. S5).It was found that S was >0, ranging from 4.57 to 26.68 (Tab. 3). This result indicates that individual processes are not acting separately, but that there is a two-way synergy between them.
| PAH | 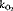 |
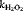 |
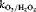 |
S (%) | PAH | 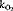 |
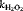 |
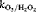 |
S (%) |
|---|---|---|---|---|---|---|---|---|---|
| Naph | 0.0045 | 0.0017 | 0.0071 | 12.32 | BaA | 0.0040 | 0.00086 | 0.0064 | 24.57 |
| Acy | 0.0046 | 0.0012 | 0.0067 | 13.22 | Chr | 0.0059 | 0.00069 | 0.0069 | 4.57 |
| Ace | 0.0033 | 0.0011 | 0.0052 | 15.33 | BbF | 0.0048 | 0.00076 | 0.0068 | 18.55 |
| Flu | 0.0038 | 0.0013 | 0.0054 | 5.54 | BkF | 0.0054 | 0.00058 | 0.0069 | 13.21 |
| Phe | 0.0033 | 0.00089 | 0.0057 | 26.68 | DBA | 0.0052 | 0.00042 | 0.0059 | 5.25 |
| Ant | 0.0043 | 0.00076 | 0.0062 | 18.34 | BaP | 0.0040 | 0.00032 | 0.0055 | 21.33 |
| Fluo | 0.0047 | 0.00082 | 0.0060 | 8.63 | Indp | 0.0051 | 0.00031 | 0.0064 | 15.25 |
| Pyr | 0.0038 | 0.00071 | 0.0061 | 26.58 | BgP | 0.0054 | 0.00029 | 0.0068 | 16.76 |
Additionally, there was no significant difference in the degradation of PAHs in the presence and absence of UV irradiation, the irradiation range was from 210 to 270 nm with a dominant wavelength of 235.7 nm (Supporting Information Fig. S7). This may be the result of the black coking sludge covering the surface of the quartz tubes which gradually prevents the spread of UV light.
3.5 Effect of pH of the sludge
 formation was increased as the pH value increased, which increased the degradation of the PAHs; and (2) the dissociation of PAHs under alkaline conditions enhanced their degradation by O3, because O3 reacts with dissociated PAHs much faster than with undissociated ones. The pH of the solution significantly influences ozone decomposition in water. Ozone decomposition proceeds with the following five-step chain reaction, as is shown in Eqs. 9-13 40. These equations show that alkaline conditions can increase ozone decomposition to form free radicals such as hydroxyl radicals (
formation was increased as the pH value increased, which increased the degradation of the PAHs; and (2) the dissociation of PAHs under alkaline conditions enhanced their degradation by O3, because O3 reacts with dissociated PAHs much faster than with undissociated ones. The pH of the solution significantly influences ozone decomposition in water. Ozone decomposition proceeds with the following five-step chain reaction, as is shown in Eqs. 9-13 40. These equations show that alkaline conditions can increase ozone decomposition to form free radicals such as hydroxyl radicals ( ), superoxide
), superoxide  , and ozonide ions
, and ozonide ions 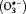 . These radicals are formed both in water and on the particle surfaces at a wide range of pH values. The target PAHs thus decomposed at an alkaline pH due to the formation of a variety of free radicals in the O3 FBR. The pH value is thus an important factor in the practical application of this method.
. These radicals are formed both in water and on the particle surfaces at a wide range of pH values. The target PAHs thus decomposed at an alkaline pH due to the formation of a variety of free radicals in the O3 FBR. The pH value is thus an important factor in the practical application of this method.
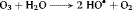 (9)
(9) (10)
(10)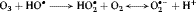 (11)
(11) (12)
(12)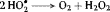 (13)
(13)
4 Concluding remarks
This study has successfully achieved sludge ozonation using an O3 FBR to reduce the amount of activated coking sludge and degrade PAHs. The process is affected by the concentration of O3, H2O2, and the pH value. The adsorption of the target PAHs on activated coking sludge greatly restricts their degradation during ozonation. The degradation kinetics of the target PAHs follows a pseudo first-order model. In the future, the impact of sludge ozonation on further persistent organic contaminants will be studied.
Acknowledgments
This research was supported by the Key Program of the National Natural Science Foundation of China (21037001); The Research Project of Production, Education and Research of Guangdong Province, P. R. China (2012B091100450) and the Fundamental Research Funds for the Central Universities (2013ZP0009).
The authors have declared no conflict of interest.




