Plant Species Richness Increased Belowground Plant Biomass and Substrate Nitrogen Removal in a Constructed Wetland
Abstract
The effects of plant species richness on both above- and belowground plant biomass, plant nitrogen (N) pool size, and substrate N concentrations were studied in a full-scale subsurface vertical-flow constructed wetland (CW). Results showed that (i) plant species richness increased belowground plant biomass and its N pool size but had no effect on aboveground plant biomass and its N pool size; (ii) plant species richness increased substrate N removal, especially ammonium N removal; and (iii) plant species richness had no effect on plant N use efficiency, suggesting that the N pool size increased with increasing plant species richness. More N accumulation could be removed through harvesting plant biomass. We concluded that the N removal performance of the CW improved by plant species richness through increasing belowground biomass and relevant N pool size.
Abbreviations:
CW, constructed wetland; DT, deviation; NUE, N use efficiency; SR, species richness
Introduction
Constructed wetlands (CWs) are engineered systems that are designed and built to utilize plants, soils, and their associated microbial assemblages to treat wastewater 1. The CW emerged as an alternative to wastewater treatment plants, with CWs being more economical and providing more ecosystem services than the conventional wastewater treatment plants in nitrogen (N) removal 2. However, the average removal rates of ammonium and nitrate were 59.8 and 14.1%, respectively 3. Improving N removal capacity is the major target in the design and operation for CWs 4.
In CWs, two main mechanisms for the N removal are plant N uptake and microbial denitrification 1, 5. Plant uptake usually plays a relative minor role, accounting for 5–23% of the total N removal 6-8. However, plants can also play important roles for they can affect both nitrification and denitrification intensities through releasing organic compounds and oxygen, and providing a suitable environment for microbes 6, 9, 10. Certain plant species may have a preference and different tolerance to NO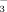 or NH
or NH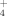 in hydraulic habitat 5, 11, thus a community with diverse plant species may have a complementary effect in different N chemical usage. Some studies showed that plants productivity (especially belowground) and species richness affected the N transformation and N removal 12, 13. Higher species richness may increase the diversity of plant root types and functions 9, 14, 15.
in hydraulic habitat 5, 11, thus a community with diverse plant species may have a complementary effect in different N chemical usage. Some studies showed that plants productivity (especially belowground) and species richness affected the N transformation and N removal 12, 13. Higher species richness may increase the diversity of plant root types and functions 9, 14, 15.
The relationships between plant diversity and biomass production 15-18 and N processes 19, 20 have been studied in grasslands. Plant biomass production was responded positively to plant species richness in many studies 18-22. Several studies found that increasing plant species richness decreased the soil N concentrations in grasslands 19, 20. The plant N pool and N use efficiency (NUE), which defined as the amount of biomass produced per unit of N uptake from the substrate 21, were responded positively to plant species richness 20, 23.
Recently, the relationships between plant diversity and removal functions in CWs have received some attention 24-26, while the results are variable. Some studies in CWs found that the plant biomass was increased with increasing species richness 24, 25, 27, while it was not related to plant species richness in other study 28. Plants in mixtures were superior in N removal rates 4, but other study found no difference in pollutant removal efficiencies of two plant mixtures 29. Some studies showed that pollutant (especially for N) removals in CWs are closely related to plant roots and root exudates 9, 13. The N bioaccumulation capacity depended mainly on plant productivity (especially belowground biomass) had been reported in some studies 30-32. The high belowground plant biomass could increase the N uptake and interactions between plants and microbes 11, 33. Some works have showed that belowground plant biomass can improve the enzyme activities related to nitrification and denitrification 13, which can promote the intensity of nitrification and denitrification in the rhizospheres 31, 33, 34. However, there is a lack of information available on how plant species richness influences belowground plant biomass and N removal functioning in CWs. The relationship between plant species richness and its productivity cannot be fully understood without paying attention to belowground plant components 35.
Our hypothesis was that the increased plant species richness may decrease N concentrations in the CW substrate through the increased belowground plant biomass and plant root uptake for N nutrients. The objectives of this study were to determine: (i) whether there was a relationship between plant species richness and belowground plant biomass in the CW; (ii) whether there was a relationship between plant species richness and the N removal in the CW.
Materials and methods
Site description and experimental design
In 2005, a subsurface vertical-flow CW (1000 m2) was established at Zhoushan City, Zhejiang Province in China (29°53′N, 122°23′E). The subsurface vertical-flow CW was filled with a three-layer filter: 0.5 m of 50–120 mm gravel in the bottom followed by 0.2 m of 6–12 mm gravel in the middle and a 0.4 m layer of coarse 1–2 mm sea sand at the top 25. The structure (50 m × 20 m × 1.2 m, length × width × depth) is outlined in Fig. 1. The CW was fed with post-treatment sewage, which had an average concentration (semimonthly measurement of 12 months, mg L−1) of 40.5 ± 5.78 of total N, 4.7 ± 0.96 of total phosphorus, 73.4 ± 9.21 of total suspended solids (SS), 19.7 ± 3.34 of chemical oxygen demand (COD), and 13.4 ± 5.11 of biochemical oxygen demand in 5 days (BOD5). The wastewater with peculiar characteristics (relatively low COD, BOD5, and SS concentrations and high nutrient concentrations) and was supplied three times per day intermittently. The CW system was designed to receive a hydraulic loading of 2000 m3 day−1 25. And the CW was supplied with 147.6 and 261.8 g N m−2 year−1 of nitrate and ammonium, thus providing a system with high N supply.

Schematic drawing of the subsurface vertical-flow CW established in Zhoushan City. The plant diversity experiment was conducted in pond A. (a) Structure of the CW, (b) designs of plant species mixtures in the pond A. At the surface of the pond A, the small black points on the pipes represent wastewater discharging holes, and the empty rectangles represent treatments planted with species richness, the numbers in the empty rectangles indicate the plant species number grown in different plots.
Sixteen plant species were selected in the experiment, which included Phragmites australis (Cav.) Trin. ex Steud. and Arundo donax L. (C3 grasses); Imperata cylindrica (L.) Beauv., Sapindus mukorossi Gaertn., Neyraudia montana Keng, Miscanthus sinensis Anderss., Triarrhena sacchariflora (Maxim.) Nakai, and Saccharu arundinaceum Retz. (C4 grasses); Canna indica L., Lythrum salicaria L., Thalia dealbata, and Cyperus alternifolius L. (forbs); and Cassia tora L., Campylotropis macrocarpa (Bunge) Rehd., Indigofera pseudotinctoria Mats, and Lespedeza bicolor Turcz. (legume species). The species are functionally and morphologically different. They are commonly used or have potential use prospects in CWs of China. In April 2006, each plot was planted at a density of 10 plants/m2 following the experimental design of Tilman et al. 17. All shoots of the planted seedlings were of similar length to ensure similar initial biomass. Experimental species composition was established by independent random draws (Fig. 1b). The distribution of species compositions was provided in Zhang et al. 25. The plots were planted to contain 1, 2, 4, 8, or 16 species. The experiment included 66 plots of 2 m × 2 m. The number of plots planted with 1, 2, 4, 8, and 16 species was 16, 10, 16, 15, and 9, respectively. To avoid confounding the effects of diversity with species identity, each diversity level had many random species compositions 18, 36. Fill planting was conducted when a plant did not survive after the initial planting. Plot compositions were maintained by hand-weeding (four or five times annually) and plots were cut all the aboveground plant in spring before growth began.
The plant diversity experiment was conducted in pond A with down-flow (aerobic condition) while pond B (anaerobic condition) was not used in the experiment (Fig. 1a).
Irrigation water level was 30 cm below the surface of the down-flow chambers (pond A), providing a uniform mesophytic habitat. The input wastewater of the sampling plots mixed in the underground at the depth of 40–50 cm. In order to maintain the substrate samplings independent, the samplings were collected in the center of each plot at the depth of 0–30 cm. Zhu et al. 24 collected samples and measured aboveground biomass and substrate N concentrations in September 2007. In this study, we focus on the effect of plant species richness on ecosystem functions through time. At the end of September 2008, samples were collected and measured for both above- and belowground plant biomass, plant N pool size, and substrate N concentrations in the CW.
Each species in monoculture under the same condition was also grown with three replicates per species. To keep the number of different species richness similar, these monoculture data were only used for calculating the ET values (the expected values of substrate nitrate and ammonium pools for each plot). And these plots were not included in the 66 plots.
Measurement and calculation
Plant biomass
To measure of net primary productivity, aboveground living plant biomass was cut at 5 cm above the surface at the end of September 2008. Plant materials were harvested in three parallel and evenly spaced 0.3 m × 2 m strips, in the center of each plot. Samples were separated into species, dried at 65°C to constant weight and weighed. Biomass values for all species in a plot were summed to obtain community biomass (g m−2).
Belowground plant biomass was collected after the harvest of aboveground plant biomass. Three soil cores per species (20 cm diameter, 40 cm deep) in the plot, by placing the plant at the center of the core, were extracted to collect roots. Root samples were sorted by species and dried at 65°C to constant weight and weighed. Root weight of each species was calculated by multiplying average root of each species weight by the number of individuals in the plot. Total biomass was calculated as the sum of above- and belowground plant biomass.
To test whether the differences in plant biomass/N ratios (= estimate of biomass produced per unit N) of a community were related to different biomass proportion of species 37, the proportion of biomass of each species in mixtures was compared between 2008 and the initial values at the start of the experiment (transplanted proportion of each species). If the species with higher biomass/N ratio also had higher biomass proportions than expected from the transplanted plants, the whole community will achieve a high biomass/N ratio 37.
Plant nitrogen pools
To determine N concentrations in plant biomass, plant samples were ground with a mini-mill “Pulverisette 23” (FRITSCH, Germany) using a 0.5-mm screen for chemical analysis. Ten to twenty milligrams of the ground plant material were used for analysis with an elemental analyzer (Vario EL Element Analyzer, Elementar, Germany). Plant community N pools were calculated by multiplying N concentrations per species by its biomass 37.
Tissue N concentrations per plot and species were used to assess the biomass/N ratios (g g−1 N), as indicators for N acquisition and use 38. Biomass/N ratios are often used as an estimate of NUE 23, 38.
Substrate nitrogen
Substrate was sampled from 0 to 30 cm depth in the center of all plots in September of 2008. Five soil cores (50 mm diameter) from each plot were mixed to form a composite sample which was then sieved to remove roots. Samples were air-dried at room temperature, and then extracted with 1 mol L−1 KCl on an orbital shaker (200 rpm) for 1 h, filtered using ash-free filter papers (pore size 0.45 µm). The extracts were measured for ammonium and nitrate concentrations using a Segmented Flow Analyzer (SAN plus, Skalar, The Netherlands).
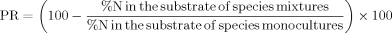 ()
()Deviation from expected substrate nitrogen
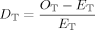 ()
()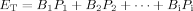 ()
()The index DT NO3 or DT NH4 < 0 indicates that mixtures were removing substrate nitrate or ammonium pools to lower levels than would be expected from the corresponding monocultures 19.
Statistical analysis
In this experiment, all analysis of variance (ANOVA) and regressions were analyzed using SPSS Version 16.0 (SPSS, Chicago, IL, USA). Effects of plant species richness and presence of legumes, C3 grasses, C4 grasses, and forbs on community above- and belowground N pool sizes and plant biomass, and biomass/N ratio were analyzed using ANOVA general linear models procedure. Linear regression models were used to identify correlations between plant species richness and above- and belowground plant biomass, as well as substrate nitrate and ammonium concentrations. Differences among plant species richness in substrate inorganic N removal rate were using one-way ANOVA by Tukey HSD tests (at p = 0.05). All statistical significance was p = 0.05 unless otherwise noted.
Results and discussion
Plant species richness increased belowground biomass production
Recently, many studies on N limited grasslands showed that plant species richness had no effect on belowground plant biomass production 18, 40, 41. However, some N addition studies showed that plant species richness had positive effects on belowground plant biomass production 42, 43. The availability of N in the ecosystem may affect the relationship 44, however, the influence of plant species richness on belowground plant biomass in CWs under high N supply remains unknown. In this study, when the N supply level was extended to 409 g N m−2 year−1, i.e., the increased plant species richness led to a significant increase in the belowground plant biomass (Tab. 1 and Fig. 2). The reason was that the presence of specific functional groups (C3 grasses) may have greater impact on belowground plant biomass (p < 0.05, Tab. 1). The increased investment in roots in a high N condition could be explained in terms of luxury consumption of nutrients (the absorbance of nutrients in excess of the immediate plant growth requirements) 45, 46.
| Source of variation | df | Nitrogen pool | Biomass | Biomass/N ratio | |||
|---|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | F | p-Value | ||
| Aboveground | |||||||
| Species richness | 4 | 0.87 | 0.490 | 0.71 | 0.591 | 0.21 | 0.940 |
| Presence of legumes | 1 | 0.85 | 0.361 | 1.44 | 0.240 | 0.17 | 0.041↓ |
| Presence of C3 grasses | 1 | 4.59 | 0.031↑ | 3.91 | 0.041↑ | 1.55 | 0.221 |
| Presence of C4 grasses | 1 | 1.84 | 0.180 | 0.67 | 0.420 | 0.03 | 0.860 |
| Presence of forbs | 1 | 1.76 | 0.191 | 0.86 | 0.361 | 0.01 | 0.931 |
| Belowground | |||||||
| Species richness | 4 | 6.38 | 0.001↑ | 7.24 | 0.001↑ | 0.79 | 0.540 |
| Presence of legumes | 1 | 0.99 | 0.320 | 0.01 | 0.920 | 0.63 | 0.050↓ |
| Presence of C3 grasses | 1 | 1.59 | 0.041↑ | 1.27 | 0.040↑ | 2.04 | 0.161 |
| Presence of C4 grasses | 1 | 0.15 | 0.711 | 0.07 | 0.791 | 1.33 | 0.031↑ |
| Presence of forbs | 1 | 0.01 | 0.920 | 0.03 | 0.870 | 0.11 | 0.740 |
- Arrows indicate significant increases (↑) or decreases (↓) of the measures with the species richness or the presence of a certain functional group. Significant p values (p < 0.05) are in bold.
- Note: About 10% of the aboveground biomass data has been used in 49.
Effects of the species richness on plant aboveground (AG) nitrogen pool size (a), plant below-ground (BG) nitrogen pool size (b), AG biomass (c), BG biomass (d), AG biomass/N ratios (e), and BG biomass/N ratios (f), respectively. Lines show predicted values from regression models.
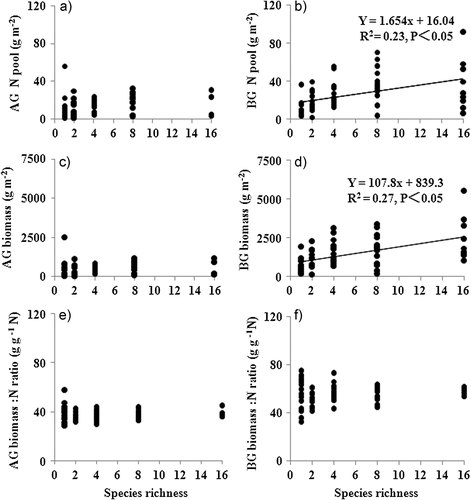
The relationships between plant species richness and aboveground plant biomass productivity were frequently positive in the studies of N limitation grasslands or wetlands 25, 40, 47, 48. However, sometimes no relationships were found under the N limitation grasslands 40, 44. When N supply was high, aboveground plant biomass was positively affected by plant species richness in the second year after plant cultivation by means of the selection effect (more diverse communities had a greater chance of containing a very productive species) 24. However, in the third year after cultivation, this study found that the aboveground biomass was not related to plant species richness (Tab. 1 and Fig. 2). The result suggested that the relationship between plant species richness and aboveground plant biomass varied over time 37, 44. The reason was that the potentially dominant species (e.g., A. donax, S. arundinaceum; Fig. 3) became the dominant species in CWs under high N supply through time; the selection effect becomes stronger over time 15. The plants trade off allocation of biomass from above to belowground in communities with high plant diversity may be another explanation 11.
Species biomass expressed as a proportion (mean ± SE) of the total community biomass in 2008 (black bars) and the plant transplanted proportion per species richness level in 2006 (white bars).
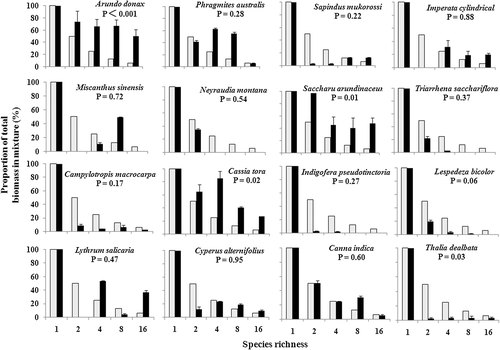
Plant species richness increased substrate inorganic N removal
Plant species richness decreased substrate inorganic N concentrations under N limitation conditions 16, 19, 20, 37. This effect was also found under some N addition studies 35, 44. However, when the N supply was high, substrate nitrate concentrations were positively affected by plant species richness 24. It might have been caused by the high N input in CW and denser plant roots 24. In this study, both substrate ammonium and nitrate concentrations decreased with the increased species richness (p < 0.05, Tab. 2 and Fig. 4). The DT NO3 and DT NH4 were significantly lower than zero (p < 0.01, Fig. 4), which indicated that plant mixtures could increase substrate nitrate or ammonium removal than monocultures 19, 27, 37. The removal rates of substrate inorganic N in plant mixtures were higher (increased by 27.98–56.12%) than those in the monocultures (Tab. 3). The finding suggested that a high species richness can enhance nitrate and ammonium removal in CWs, thus confirming our hypothesis. The relationship between plant species richness and the N removal from the substrate may vary over time 44. Dense plant roots may increase the availability of oxygen and provide more exudates 5, 6, 9, 25 that may improve the nitrification in the substrate 9, 33, 50. And the high ammonium concentration in the substrate may also improve the nitrification 51. Furthermore, the greater rhizome and dense plant roots increase the N uptake and incorporation by plants and microbes 11, 52, 53.
| Response | Variable | F | p-Value | R2 |
|---|---|---|---|---|
| Nitrate (mg kg−1) | Species richness | 3.89 | 0.040↓ | 0.27 |
| Aboveground plant biomass | 3.18 | 0.041↓ | 0.14 | |
| Belowground plant biomass | 1.44 | 0.240 | 0.02 | |
| Ammonium (mg kg−1) | Species richness | 3.45 | 0.031↓ | 0.38 |
| Aboveground plant biomass | 1.35 | 0.091 | 0.02 | |
| Belowground plant biomass | 7.23 | 0.011↓ | 0.34 |
- Significant p values (p < 0.05) are in bold. Arrows indicate significant increase (↑) or decrease (↓) of the measures with each variable.
Relationships among DT NO3 (a), DT NH4 (b), and substrate dissolved inorganic N (DIN) concentrations (c), and species richness, respectively. DT NO3 or DT NH4 < 0 indicates lower substrate nitrate or ammonium pools in mixtures compared to the average of the component monocultures. Lines show predicted values from regression models.
| Species richness | NO3–N (mg kg−1) | NH4–N (mg kg−1) | IN (mg kg−1) | NO3–N removal (%) | NH4–N removal (%) | IN removal (%) |
|---|---|---|---|---|---|---|
| SP1a | 2.03 ± 0.50a,b | 3.57 ± 0.26a | 5.60 ± 0.47a | −c | – | – |
| SP2 | 1.67 ± 0.12ab | 2.36 ± 0.09b | 4.03 ± 0.03b | 17.64 ± 4.12 | 33.87 ± 8.21 | 27.98 ± 9.26 |
| SP4 | 1.44 ± 0.52ab | 2.24 ± 0.94b | 3.67 ± 0.14bc | 29.33 ± 3.26 | 37.41 ± 10.06 | 34.47 ± 5.58 |
| SP8 | 0.87 ± 0.36b | 2.12 ± 0.86b | 2.99 ± 0.12c | 57.34 ± 6.28 | 40.61 ± 11.14 | 46.67 ± 8.81 |
| SP16 | 0.98 ± 0.27b | 1.48 ± 0.71c | 2.46 ± 0.83c | 51.89 ± 9.18 | 58.54 ± 15.56 | 56.12 ± 14.64 |
- a SP1, 2, 4, 8, and 16 represent five species richness levels with 1, 2, 4, 8, and 16 species.
- b The same lowercase letters in a column indicate non-significant differences among the species richness levels.
- c The monocultures, as the control, were calculated as Eq. (1) make no sense.
Although the mechanism for removal of nitrate and ammonium from domestic wastewater in the CWs were unclear 52, this study showed that high species richness may promote plant uptake and nitrification and can enhance nitrate and ammonium removal in the CW. Zhang et al. 54 found that the effluent inorganic N concentrations closely related to substrate inorganic N concentrations. The decrease of both substrate ammonium and nitrate concentrations with the increased plant species richness (p < 0.05, Tab. 2 and Fig. 4) suggested that high species richness could enhance effluent N removal in the CW.
Plant species richness enhanced plant N uptake but did not influence NUE
Plant N pool size increased with the increased plant species richness in some studies of N limitation grasslands or wetlands 11, 20, 27, 43. Although lower N availability probably flattened the relationship between plant species richness and plant N pools 37, the influence of plant species richness on plant N pools under high N supply remains unknown. This study found plant belowground N pool size was positively correlated with plant species richness under high N supply (p < 0.05, Tab. 1 and Fig. 2), confirming further our hypothesis. The increase in plant N pool size may be caused by enhancement of rhizosphere effects in more diverse mixtures, which increased water retention and then allow plants to maximize uptake of N 55. High biomass production in high diverse mixtures also increased the plant N accumulation 27, 30, 56, 57. This study found that plant N uptake was positively related to plant species richness even under high N supply via belowground plant components. It extended the theories of species richness-plant N pool from low N natural ecosystem to higher N input ecosystem.
In this study, the presence of legumes and C4 grasses decreased and increased above- and belowground plant biomass to N ratio (p < 0.05, Tab. 1), respectively. In general, C4 grass species had the largest biomass/N ratios in comparison to forbs and C3 grasses. Legumes had the lowest biomass/N ratios (Fig. 5), because high substrate N availability often suppresses N2 fixation 37. The results suggested a large variation in resource acquisition strategies among different functional groups 38. Some studies of N limitation grasslands found that increasing plant diversity promoted NUE of plants 23, 37. But this high N supply study showed that plant NUE (biomass/N ratio) was not affected by plant species richness (p > 0.05, Tab. 1 and Fig. 2). It might be because that the plant community not only consisted of C4 grasses species but also consisted of legumes (Fig. 5). The effects of plant species richness on NUE might be offset by the mixture of these plant species in the CW. It showed that increased plant species richness increased in N uptake rather than NUE under high N supply. Thus, high N accumulation in more diverse mixtures could be beneficial for N removal through frequent harvesting plant biomass in CWs 6-8.
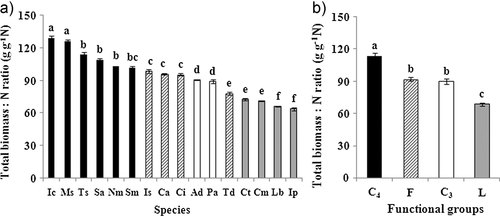
Biomass/N ratios (mean ± SE) of each species (a), and functional groups (b) averaged across all mixtures based on the harvest in October 2008. Different letters above bars in (a), and (b) indicate significant differences among species and functional groups (p < 0.05). Abbreviations of species names: Ad, A. donax; Pa, P. australis; Sm, S. mukorossi; Ic, I. cylindrica; Ms, M. sinensis; Nm, N. montana; Sa, S. arundinaceum; Ts, T. sacchariflora; Cm,C. macrocarpa; Ct, C. tora; Ip, I. pseudotinctoria; Lb, L. bicolor; Ls, L. salicaria; Ca, C. alternifolius; Ci, C. indica; Td, T. dealbata.
Conclusions
The study showed that the increased plant species richness improved the belowground plant biomass and its N pool size, and decreased substrate inorganic N concentrations in the subsurface vertical-flow CW. However, the increased plant species richness did not affect the NUE (biomass/N ratio), indicating that increased in N uptake rather than NUE under high N supply. Our data indicated that belowground plant components played very important roles in N removal. In conclusion, the increasing species richness can increase N removal in CWs by enhancing plant belowground biomass and N uptake.
Acknowledgements
This research was supported by funding from the National Science Foundation of China (grant number 31170305 and 31270377). S. X. Chang was supported by a Discovery Grant from the Natural Science and Engineering Research Council of Canada (NSERC). We also thank Dr. D. Liu, H. L. Ge, and H. Q. Cao for their help in this study.
The authors have declared no conflict of interest.



 /NO
/NO Ratio Affect Ryegrass (Lolium Perenne L.) Growth and N Accumulation in a Hydroponic System
Ratio Affect Ryegrass (Lolium Perenne L.) Growth and N Accumulation in a Hydroponic System /NH
/NH Ratios Affect the Growth and N Removal Ability of Acorus calamus and Iris pseudacorus in a Hydroponic System
Ratios Affect the Growth and N Removal Ability of Acorus calamus and Iris pseudacorus in a Hydroponic System
