Pathology primer: Common liver biopsy findings in patients who have recently undergone liver transplant or resection
Potential conflict of interest: Nothing to report.
Abbreviations
-
- AMR
-
- antibody-mediated rejection
-
- CMV
-
- cytomegalovirus
-
- DSA
-
- donor-specific alloantibody
-
- HAT
-
- hepatic artery thrombosis
-
- HV
-
- hepatic vein
-
- POLF
-
- postoperative liver failure
-
- PT
-
- portal tract
-
- PVE
-
- portal vein embolization
-
- SFSS
-
- small-for-size syndrome
-
- TCMR
-
- T cell–mediated (acute cellular) rejection
Two general sources of liver biopsy exist in the postoperative period: one is the “biopsy” of the parenchyma background to a resected neoplasm, and the other is needle biopsy in response to postoperative liver dysfunction. This review is discussed along the following outline: (1) biopsy to address postoperative liver dysfunction, such as sepsis, inadequate residual volume, drug-induced toxicity/hypersensitivity, vascular/ischemic injury (e.g., hepatic artery thrombosis [HAT]), and biliary complications; (2) background liver abnormalities in tumor resections, such as microscopic metastases, preoperative embolization, effect of neoadjuvant chemotherapy and/or radiation therapy, underlying chronic parenchymal disease, and “surgical hepatitis”; and (3) common liver biopsy findings in the early posttransplant period, such as preservation/reperfusion (ischemic) injury, HAT, biliary obstruction, infections (e.g., cytomegalovirus [CMV]), T cell–mediated (acute cellular) rejection (TCMR), and antibody-mediated rejection (AMR).
Postoperative Liver Dysfunction
Postoperative liver failure (POLF) could occur in 0.7% to 9.1% of cases.1 The status of the underlying parenchyma in the resected specimen is an important histopathological variable and is discussed in the next section. When POLF triggers a needle biopsy, sepsis, drug reaction, inadequate residual liver volume, and ischemia represent the major issues that have been observed in this context.
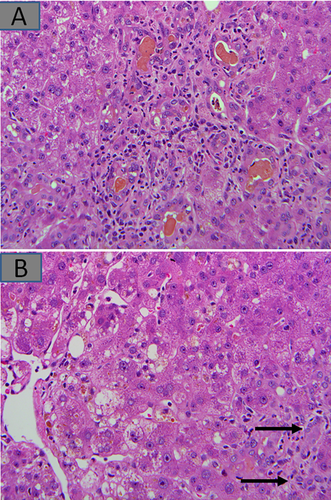
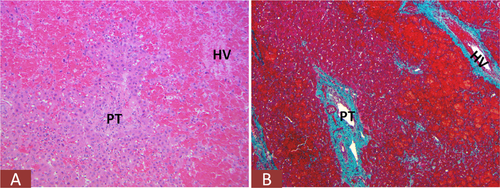
Postoperative sepsis, either intra-abdominal or systemic, could manifest as mild portal inflammation, sometimes but not always neutrophil-rich, as well as hepatocanalicular and ductular cholestasis, sometimes referred to as cholangitis lenta2 (Fig. 1A). These features are not specific, and the differential diagnoses in the appropriate context include small-for-size syndrome (SFSS), parenteral nutrition, and reaction to anesthetic and other mediations including antibiotics. SFSS refers to inadequate postresection parenchymal volume. In these cases, too much portal venous flow into a small liver results in liver sinusoidal hyperperfusion followed by buffer reduction in hepatic arterial flow, leading to parenchymal and sinusoidal endothelial injury, as well relative or absolute bile duct ischemia.3, 4 The features of small residual volume therefore include hepatocellular swelling, cholestasis, and mild inflammation and could overlap or coexist with those of sepsis. Ductular cholestasis is not common, but ischemic-looking bile ducts from relative arterial insufficiency could be seen (Fig. 1B). Preexisting liver disease is an important determinant of postresection parenchymal adequacy (discussed later).
Several medications including anesthetic agents and antibiotics used in the postoperative period could result in liver injury. Liver injury by drugs, including those used in the perioperative period, was recently reviewed in Clinical Liver Disease.5 Importantly, the patterns such as cholestasis, hepatitis, and others become clear with detailed review of the drug list and relevant temporal associations.
Perioperative ischemic injury resulting from global hypovolemia (shock) or local hepatic hypoperfusion is an important cause of postoperative morbidity. Ischemic liver injury is characterized by zone 3–centered bland necrosis with varying degrees of hepatocellular injury toward the zone 1 areas (Fig. 2A). A different kind of hemodynamic complication is venous outflow obstruction from hepatic venous and/or caval thrombosis. In these cases, marked congestion with occlusion of intrahepatic hepatic vein (HV) branches is seen.
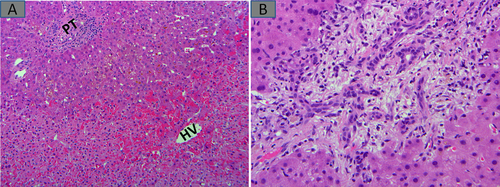
Lastly, nonischemic mechanical injury to the biliary tree could occur, especially in surgeries that require biliary reconstruction. Depending on the degree and level of these injuries, liver functional abnormalities could present anytime from the immediate postoperative period to several weeks or months. It is not uncommon for postresection patients to return to the operating room for diagnostic or therapeutic laparotomies with subsequent wedge biopsies (Fig. 2B).
Background Liver Abnormalities in Tumor Resections
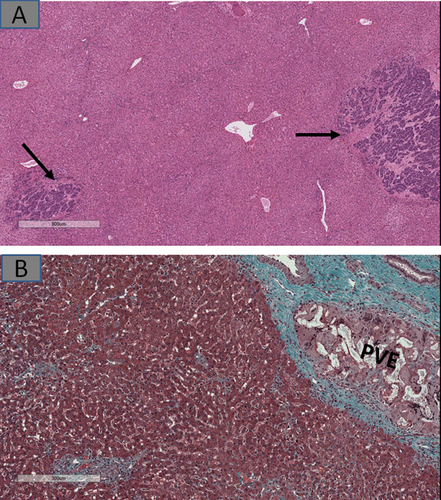
The presence of microscopic metastases (Fig. 3A), which might have been invisible in preoperative imagings, could imply similar lesions are present in the unresected liver. Also, in patients who had preresection portal vein embolization (PVE) meant to force hypertrophy of residual liver lobes, embolization material in the portal vein branches could be accompanied by parenchymal ischemia (e.g., atrophy, patchy fibrosis, or pseudosinusoidal dilatation) and/or nondescript nodularity (Fig. 3B).
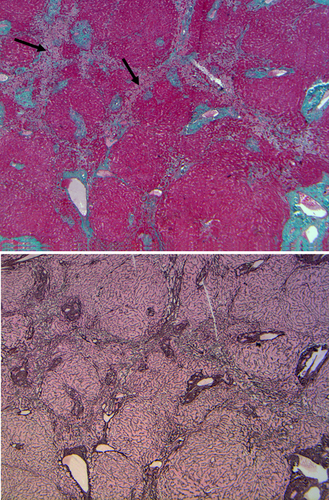
The impact of neoadjuvant chemotherapy or radiation therapy could be seen in the resected liver parenchyma. These vary depending on the chemotherapeutic or radiation methods used and could include steatosis/steatohepatitis, microvascular injury with (Fig. 4) or without (Fig. 5) sinusoidal obstruction.6, 7
A previously unknown liver parenchymal disease found in a resection could often explain postoperative liver function abnormalities. A recent US survey showed that 69% of cirrhotics were unaware they had liver disease.8 In addition to cirrhosis, other abnormalities that might not have been uncovered preoperatively could therefore provide vital information, such as steatohepatitis, parasites, previously undiagnosed metabolic diseases such as hemosiderosis (Fig. 6A), and others.

Surgical hepatitis is mentioned in this review to emphasize that this is not an abnormality that requires intervention. This manifests in resected livers as aggregates of neutrophils responding to extended pretransection intraoperative manipulations. Many pathologists, rightly, would not mention this in the report for this reason. When it occurs in a steatotic liver, its presence should not be the basis for diagnosing steatohepatitis (Fig. 6B).
Common Liver Biopsy Findings in the Early Posttransplant Period
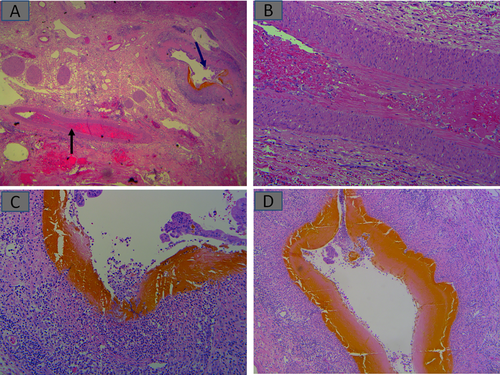
Ischemia from organ harvesting and preservation occurs because of alternating cold and warm ischemic periods, followed further by insults from restoration of blood flow (reperfusion injury). These injuries are, however, indistinguishable from other forms of ischemic insults highlighted earlier in Fig. 2A. In patients with marginal grafts from steatosis, deceased cardiac donors, or other reasons, these could be severe enough to cause delayed graft function. Ischemia that affects the biliary tree manifests “upstream” in needle biopsies as biliary obstruction (Fig. 2B) as a result of sludge-filled “downstream” necrotic large bile ducts (Fig. 7A). An additional source of biliary and parenchymal ischemia could result from HAT. This scenario often requires early diagnosis and intervention (Fig. 7B). Biliary obstruction occurring very early is more likely to occur as part of an ischemic injury, including HAT, as discussed earlier. However, early-onset anastomotic strictures have also been described.
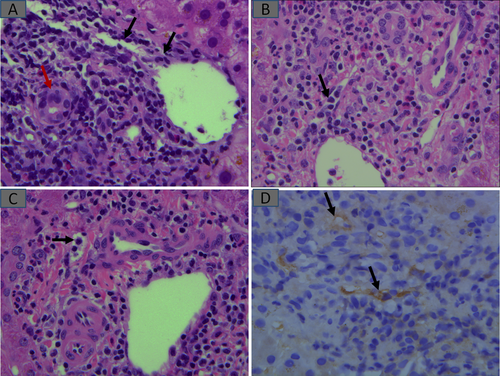
Infections and alloimmune injury occur opposite of two extremes and frequently require biopsies to resolve. For example, CMV infection usually presents very early, as is the case with rejection. TCMR and/or AMR alloimmune injuries are important indications for posttransplant biopsies. TCMR is recognized as inflammation directed at bile duct epithelium and portal and HV endothelial cells. As such, inflammation is limited to sites where these structures are located, that is, portal tracts (PTs) and HV walls, resulting in bile duct injury and phlebitis (endothelialitis) (Fig. 8). AMR is increasingly recognized, although its role as a rare cause of early graft dysfunction had always been acknowledged.9 Inciting donor-specific alloantibodies (DSAs) could be anti-ABO, human leukocyte antigen, or rarely, others. Hyperacute AMR occurs immediately after reestablishment of vascular anastomosis with graft swelling, hemorrhages, and extensive vascular thromboses and fibrinoid necroses, whereas acute AMR occurs in the hours to several days posttransplant. A combination of clinical, laboratory (DSA demonstration), and histopathological parameters characterize AMR.9 Portal capillaritis (Banff h score) and C4d deposition in portal capillaries and inlet venules (with or without sinusoidal C4d) are hallmarks of AMR9 (Fig. 8B-D). Cholestasis is frequently present, most likely because of DSA-injured peribiliary capillary plexus.
Summary
Postoperative biopsies for liver functional abnormalities performed to answer specific questions could uncover a range of surgery-related complications including SFSS, ischemia, adverse drug reactions, and complications from bile duct injury, vascular outflow obstruction, and other hemodynamic abnormalities. Liver pathology in the postoperative period includes evaluation of the resected liver for underlying/preexisting pathological processes, which may not be apparent before surgery. In addition, more extensive metastases than radiologically evident should be reported. Findings relating to preoperative therapies including neoadjuvant chemoradiation, PVE, and others should be documented because they could have an impact on postoperative course. Lastly, in the liver transplantation setting, ischemic injuries from preservation, reperfusion, and other sources are important causes of early graft dysfunction, as well as TCMR and AMR alloimmune injuries, all of which have recognizable histopathological features that help with diagnoses, especially in differentiating from de novo, recurrent, or reactivated infections.