Thrombosis and coagulopathy in the liver transplant candidate and recipient
Potential conflict of interest: Nothing to report.
Abstract
Watch a video presentation of this article
Abbreviations
-
- Ag
-
- antigen
-
- ALF
-
- acute liver failure
-
- ALI
-
- acute liver injury
-
- INR
-
- international normalized ratio
-
- PVT
-
- portal vein thrombosis
-
- SOC
-
- standard-of-care
-
- TEG
-
- thromboelastography
-
- TM
-
- thrombomodulin
-
- vWF
-
- von Willebrand factor
Patients with liver disease are considered at risk for bleeding complications based on complications of portal hypertension and abnormalities in standard laboratory tests. However, evidence accumulated over the last 10 years suggests that the state of global hemostasis in patients with cirrhosis and acute liver failure (ALF) is “rebalanced,” such that compensation occurs for deficient procoagulant, liver-derived factors. Recent clinical and in vitro research has demonstrated that stable patients with acute and chronic liver failure actually exist in a relative hypercoagulable state, which may propagate the progression of liver disease itself, as well as cause thrombotic complications. Therefore, treating such patients with blood and blood products has the potential to exacerbate the hypercoagulable state and cause harm. This article reviews the basis of rebalanced hemostasis in patients with cirrhosis and ALF, and suggests therapeutic options with which to address problems of bleeding or thrombosis.
Rebalanced Hemostasis in Patients With Cirrhosis
Bleeding from complications of portal hypertension in patients with cirrhosis is primarily due to pressure within portosystemic collaterals, not from abnormalities in hemostasis. Consequently, portal hypertensive bleeding will not be further considered.
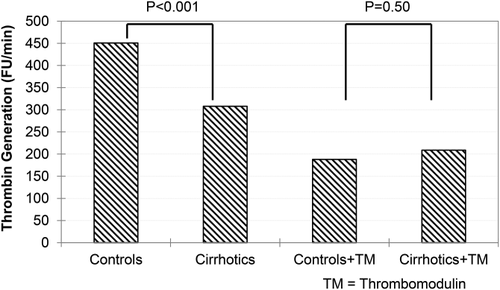
Tripodi and associates1 provided the seminal experimental evidence introducing the concept of “rebalanced hemostasis” in 2005 using an in vitro manipulation that demonstrated that thrombin generation in patients with cirrhosis was similar to that in normal healthy control subjects (Fig. 1). Although thrombin generation in cirrhotic patients was initially found to be lower than in control subjects because of lower levels of procoagulant factor synthesis (Fig. 1, two left bars), the same experiments in the presence of thrombomodulin, an endogenous activator of protein C derived from endothelial cells, showed that thrombin generation in cirrhotic patients was similar to control subjects (Fig. 1, two right bars). These experiments provided the first evidence of “rebalanced” hemostasis, in which procoagulant and anticoagulant, liver-derived coagulation factors decrease in parallel in patients with cirrhosis. Thus, thrombin generation in cirrhotic patients is similar to normal healthy control subjects, if assay conditions account for missing endothelial proteins to activate protein C (i.e., by the addition of thrombomodulin).
A second set of experiments provided a potentially important threshold for the platelet count, below which clinicians might be wary of procedure-related bleeding complications in patients with cirrhosis.2 Recognizing the importance of platelets to thrombin generation, Tripodi et al.2 performed thrombin generation assays in plasma containing normal platelet counts from normal healthy control subjects. Thrombin generation in 90% of healthy control subjects (median platelet count of 198 × 109/L) was ≥875 nmol/L. Defining this level of thrombin generation as “normal,” the investigators repeated the experiments using plasma from patients with cirrhosis into which platelets were added back to the reaction mixture in increasing numbers, and they found that a platelet count of 56 × 109/L was adequate to generate ≥875 nmol/L thrombin. These observations suggest that ∼60 × 109/L may serve as a guideline for platelet transfusion before invasive procedures in patients with cirrhosis, and also possibly a goal of transfusion. Obviously, clinical correlation is needed.
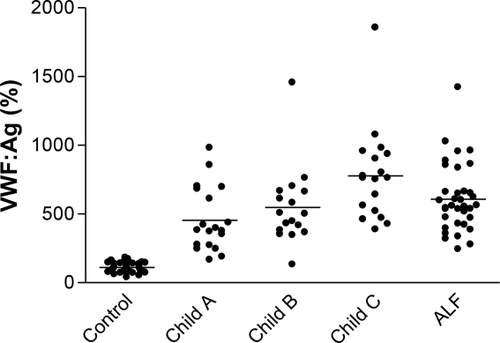
In addition to their function as activators of thrombin, platelets also serve to adhere to endothelial defects in primary hemostasis via the endothelial-derived protein, von Willebrand factor (vWF). Although patients with cirrhosis and portal hypertension typically have thrombocytopenia, they also have high levels of vWF in proportion to the severity of liver failure, which can increase platelet adherence and compensate for numerical deficiency (Fig. 2).3
Cirrhosis as a Hypercoagulable State
In contrast with the perception of a bleeding tendency, thrombosis has become increasingly recognized as a major clinical problem in patients with cirrhosis, not only in peripheral vascular beds, but also within hepatic vasculature itself. As a hypercoagulable state within the liver, thrombosis may contribute to the pathogenesis of cirrhosis and portal hypertension. Wanless et al.4 graded portal and hepatic venous micro-obliterative lesions in explanted livers and found a direct correlation with focal parenchymal extinction within the same vascular distribution. These observations suggested that thrombosis of portal and hepatic vessels propagated parenchymal collapse, worsened portal hypertension, and increased the risk for variceal bleeding.
Several clinical observations also support the presence of a systemic hypercoagulable state in patients with cirrhosis. The incidence of venous thromboembolism in patients with cirrhosis admitted to tertiary care hospitals is significant and may be higher than noncirrhotic control subjects.5 In a population-based study, unprovoked venous thromboembolism was ∼2-fold more common in patients with cirrhosis than in control subjects.6 Finally, the thrombosis of continuous renal replacement therapy circuits in patients with liver failure was more rapid and more common than in control subjects and could be delayed by anticoagulation without an obvious increase in bleeding complications.7 Thus, the concept of “auto-anticoagulation,” the notion that patients with cirrhosis are protected de facto from venous thromboembolism by virtue of elevated international normalized ratio (INR), has been strongly refuted. Studies to define the mechanisms by which some patients with cirrhosis have a hypercoagulable state are ongoing.
Management
Blood and Blood Product Transfusions in Cirrhosis
The concept of rebalanced hemostasis in cirrhosis has not been rigorously tested in clinical situations; there are no randomized, prospective studies that document the safety of not administering plasma or platelet transfusions before invasive procedures. However, clinical observations support sparing many patients blood product transfusions. An attempt to systematically explore the rational administration of blood products before invasive procedures in patients with cirrhosis was recently reported in 60 patients with significant coagulopathy, defined as an INR greater than 1.8 and/or platelet count less than 50 × 109/L.8 Patients randomized to the standard-of-care (SOC) group received plasma at the dose of 10 mL/kg of ideal body weight for INR greater than 1.8 and/or platelets in the amount of one unit if the platelet count was less than 50 × 109/L. Patients randomized to the study group received plasma or platelet transfusions only based on specific (and largely arbitrary) abnormal thromboelastography (TEG) parameters.9 As shown in Fig. 3, only 17% of patients in the TEG group received a blood product transfusion versus 100% of the SOC group (by definition), with no difference in the rare occurrence of procedure-related bleeding complications.

Treatment of the Hypercoagulable State of Cirrhosis
Nonmalignant portal vein thrombosis (PVT) occurs in up to 25% of patients with cirrhosis awaiting liver transplantation, as a result of the relative hypercoagulable state discussed earlier, as well as pooling and stagnant flow and local endothelial dysfunction within the portal vein. The risk for PVT reflects the severity of underlying cirrhosis, and its incidence increases with decompensation. Although PVT does not appear to be responsible for the progression of cirrhosis per se, its occurrence is associated with acute decompensation, variceal bleeding, and increased mortality.10 Nonocclusive is much more common than occlusive PVT, and the prevalence of the former varies with time because of spontaneous recanalization in up to 70% of cases.
A small but seminal study by Villa et al.11 randomized 70 patients with Child's B/C cirrhosis to enoxaparin (4000 IU/day) or placebo for 48 weeks to determine whether PVT could be safely prevented. The 2-year prevention of portomesenteric venous thrombosis detected by 3-month ultrasound examinations (primary endpoint) was 0% in the enoxaparin-treated group but 27.7% in the control group (P = 0.001). Perhaps more impressive were the secondary endpoints of hepatic decompensation and overall and transplant-free survival, all of which occurred with an incidence implying benefit from enoxaparin. Although platelet count decreased in the enoxaparin group by nearly 50%, there was no relation of treatment arm to the rare occurrence of bleeding complications. Perhaps the most intriguing finding of this study was the fact that the benefits of enoxaparin on rates of hepatic decompensation and survival occurred independently of PVT prevention, supporting the hypothesis that thrombosis of the hepatic microcirculation contributes to the progression of liver disease.
The treatment of existing PVT in patients with cirrhosis has been recently summarized.12 In four retrospective studies using enoxaparin, complete recanalization occurred in 42% to 75%, and no recanalization occurred in 17% to 53% of patients. Importantly, there were no bleeding-related deaths due to anticoagulation in any of these studies. The presence of PVT for fewer than 6 months may be an indication for anticoagulation, because recanalization rarely occurs later.13 Conversely, recanalization on anticoagulation usually occurs within 6 months. Insertion of a transjugular intrahepatic portosystemic shunt through a portal thrombus has also been tested to re-establish portal flow and lower portal pressure in cases where anticoagulation has not resulted in recanalization.14
Rebalanced Hemostasis in Patients With ALF
Patients with ALF are perceived to have a bleeding tendency primarily on the basis of profoundly elevated INR, which can be unmeasurably high. The INR is an important prognostic indicator in ALF, but not because it predicts bleeding complications. In fact, the INR of patients with and without bleeding complications is not significantly different over the first week after admission for ALF (ALF Study Group, unpublished data). Patients with ALF also experience thrombocytopenia,15 although, on average, to a less impressive nadir than patients with cirrhosis.
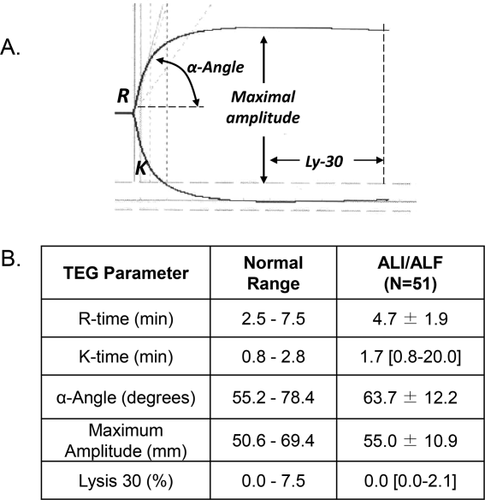
The conundrum of a perceived bleeding tendency in the face of infrequent bleeding complications in patients with ALF has been recently explored. As shown in Fig. 4, global hemostasis as assessed by TEG is usually normal.16 Two obvious contributors to hemostatic rebalance in this patient population include high factor VIII and vWF levels, a consistent finding in ALF because of activation and injury of vascular endothelium.17 In a series of 51 patients with acute liver injury (a milder form of ALF without encephalopathy) and ALF, the mean and median TEG parameters were well within normal limits (Fig. 4B). Other studies have shown thrombin generation in the presence of thrombomodulin in plasma from patients with ALF is similar to normal healthy control subjects, and these results are similar to those in patients with cirrhosis.18
ALF as a Hypercoagulable State
Similar to patients with cirrhosis, the earlier data suggest that hemostasis in patients with ALF exists in a rebalanced state slightly biased toward thrombosis. Few clinical studies of thrombotic complications in ALF exist, but several observations strongly suggest the presence of relative hypercoagulability. Hemostasis in whole blood by TEG has implied a hypercoagulable state in 25% to 35% of patients with ALF.16, 19 Renal replacement therapy circuits frequently thrombose in patients with ALF. Data in animal models of ALF have correlated the degree of coagulation activation within the liver and extent of necrosis, implying that intrahepatic thrombosis may cause a secondary ischemic hit after the primary liver injury. Furthermore, heparin alleviates acetaminophen-induced hepatotoxicity.20 Finally, patients with ALF with multiorgan system failure develop peripheral tissue hypoxia leading to lactic acidosis, caused in part by microthrombi in peripheral microcirculation. Thus, although gross thrombotic complications in ALF may not be a prominent feature of the syndrome, thrombosis of the hepatic and systemic microvasculature is likely to be a major contributor to the pathogenesis of the syndrome and to poor outcome.
Management of Hemostatic Abnormalities in Patients With ALF
There are few data to guide clinicians in using blood and blood products or anticoagulants in patients with ALF, and recommendations are primarily based on personal experience. It should be emphasized that spontaneous and postprocedural bleeding complications are uncommon in patients with ALF. When they occur, they reflect the severity of the secondary systemic inflammatory response syndrome and systemic complications rather than the severity of the primary liver injury. Consequently, they can be anticipated by a low platelet count, but not a high INR. The severity of bleeding complications in patients with ALF is usually mild and self-limited, and does not require red blood cell transfusion. However, bleeding complications portend poor outcome, most likely because of their association with multiorgan system failure, but not bleeding per se. The bleeding complication with highest morbidity and mortality in patients with ALF is intracranial bleeding after intracranial pressure monitor placement; although uncommon (∼5%), it has a high mortality (∼50%). These observations are based on extensive review of the ALF Study Group Registry of nearly 2000 patients, but they have not yet been published.
The decision to transfuse patients with ALF should not be taken lightly. Primarily, transfusion of plasma removes the most important prognostic indicator of spontaneous recovery of the liver, because the clinician can no longer rely on the trend in INR. It has also been observed in the ALF Study Group Registry that transfusion of red blood cells, platelets, or plasma is associated with a nearly 50% increase in death or liver transplantation at day 21 after admission. Although the need for transfusion of any blood component selects for more acutely ill patients, this observation also raises the possibility that transfusions cause harm. As discussed earlier, patients with ALF may be hypercoagulable, and systemic and intrahepatic activation of coagulation increases; it is therefore plausible that blood and product transfusions cause harm by exacerbating microvascular thrombosis, exacerbating not only the liver injury, but also peripheral tissue hypoxia and multiorgan system failure.
Transfusions in patients with ALF should, therefore, be reserved for clinically significant bleeding. As prophylaxis, they should be reserved for highest risk procedures, such as placement of an intracranial pressure monitor. A goal INR should probably not be used to guide transfusions; instead, ∼2 units of plasma transfused within roughly an hour before the procedure without repeating the INR might be considered, because this strategy repletes procoagulant factors to achieve a minimal level to support thrombin generation. Platelet transfusions should be considered when less than ∼60 × 109/L, based on the work described earlier, which has not yet been reproduced in patients with ALF. Fibrinogen repletion as cryoprecipitate should be considered when plasma concentrations are less than 100 mg/dL. The use of TEG to guide repletion would be reasonable, if available. Treatment of the underlying precipitating factor of the bleeding should always accompany transfusions. The precipitating factors resemble those described for cirrhosis, most importantly, infection and renal failure.
The use of anticoagulants in patients with ALF is equally based on local experience and practice. During renal replacement therapy, it has been recommended that citrate be avoided because of the decreased hepatic capacity to metabolize citrate; however, a recent report has suggested that citrate is probably safe.21 The use of heparin in renal replacement therapy is also probably safe in patients with ALF, although it may not be as effective as in other critically ill patients without liver failure because of low antithrombin levels in the former. Some form of venous thromboembolism prophylaxis should be strongly considered. Pneumatic compression devices may be more appealing to clinicians in the setting of renal failure or severe thrombocytopenia, but low-dose heparins have been used without complications (unpublished observations).
Conclusions
In conclusion, stable patients with liver disease may be considered to have rebalanced hemostasis. However, hemostasis in unstable patients with severe acute or chronic liver disease exists in a fragile state of compensation, the balance of which may be tipped toward bleeding or thrombosis by a number of precipitating factors (most importantly, infection). Unfortunately, few clinical studies have proved the safety of withholding procoagulant therapies before high-risk invasive procedures. Further studies are urgently needed to determine whether blood product transfusions cause harm in patients with severe acute and chronic liver disease, because tipping the balance toward hypercoagulability may exacerbate liver injury and systemic complications in both syndromes.