Histologic and noninvasive estimates of liver fibrosis
Potential conflict of interest: Nothing to report.
Abbreviations
-
- APRI
-
- AST-to-Platelet Ratio Index
-
- AUROC
-
- area under the receiver operating characteristic curve
-
- CLD
-
- chronic liver disease
-
- MR
-
- magnetic resonance
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- TE
-
- transient elastography
-
- US
-
- ultrasound.
The severity of liver fibrosis is important in therapeutic decision-making and in predicting prognosis of chronic liver disease (CLD). Therefore, its assessment is, and will stay, a major clinical issue in hepatology for the next coming years. Liver biopsy is still considered as the reference test, but noninvasive techniques have been progressively implemented in the clinical practice and will continue to improve with sophistication of technologies.
Liver Biopsy
Because hepatic fibrosis relies on the deposit of extracellular matrix components in optically visible structures within the liver parenchyma, biopsy is considered the reference tool because it assesses both the amount of fibrosis, its distribution within the liver lobule, and other associated lesions including architectural distortion (Fig. 1). However, liver biopsy has limitations inherent to either the procedure itself or to the lesion to be evaluated (Table 1).1-3 It is costly and is associated with risk of adverse events (pain, bleeding), including minor mortality rates. Sampling error and interobserver variation are also limitations that can be partially overcome by increasing the length of liver biopsy, using highly reproducible histopathological scoring systems, or referring to an experienced liver pathologist. Thus, although liver biopsy has limitations, appropriate precautions may partly reduce its flaws. Semiquantitative histological staging systems such as METAVIR, Ishak scores, or the Laennec score for staging cirrhosis are very useful in everyday practice but do not mirror linearity of fibrosis deposition (Fig. 2). Computer-aided morphometric measurement of collagen proportional area is a partly automated technique that provides an accurate and linear evaluation of fibrosis amount.4 Another limitation of the biopsy is that histological staging gives a snapshot of disease at that moment, and not an insight into the dynamics of the process (whether disease is in progression, static, or regression). Immunohistochemical evaluation of smooth muscle actin expression for hepatic stellate cell activation, cytokeratin 7 for labeling ductular proliferation or CD34 for visualization of endothelial capillarization, or the use of two-photon and second-harmonic generation fluorescence microscopy techniques for spatial assessment of fibrillar collagen should provide better dynamic measures.5, 6
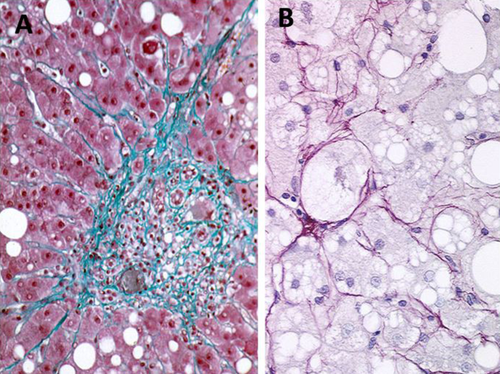
(A) Periportal fibrosis: fibrous tissue extending in the periportal area as stellate expansion of thin collagen fibers, a typical aspect of chronic viral hepatitis (Masson's trichrome staining; magnification, ×10). (B) Perisinusoidal fibrosis: Thin collagen fibers embedding ballooned hepatocytes, a characteristic pattern of NAFLD (Sirius red; magnification, ×40)
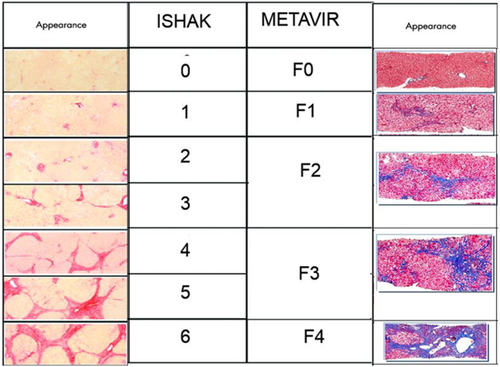
Ishak and METAVIR scoring system for fibrosis.
| Liver Biopsy | |
|---|---|
| Advantages | Gold standard for fibrosis estimate |
| Assessment of fibrosis in its histopathologic environment | |
| Semiquantitative scoring system with high interobserver agreement | |
| Accurate quantitative evaluation of collagen proportional area (morphometry) | |
| Insight in dynamic of fibrosis (immunohistochemical markers) | |
| Applicability for any type of chronic liver disease | |
| Disadvantages | Sampling error (imperfect gold standard) |
| Cost | |
| Limited access | |
| Morbidity (mortality) | |
| Serum Markers | |
| Advantages | Cheap and easy-to-use (not patented tests) |
| Good interlaboratory reproducibility | |
| High and widespread applicability | |
| Noninvasive method, can be repeated easily | |
| Obviate the need for biopsy in a sizable number of patients | |
| Disadvantages | Nonspecific of the liver (confounding factors depending on each test) |
| Evaluation based on dichotomizing outcomes | |
| Mainly validated in chronic hepatitis C | |
| Gray zone for assessment of intermediate stage of fibrosis | |
| Better performance for detecting cirrhosis than advanced fibrosis | |
| Cost associated with patented test | |
| Imaging Modalities (TE and MRI) | |
| Advantages | Global evaluation of liver fibrosis |
| Noninvasive method | |
| Fast, simple, and user-friendly (TE) | |
| Validated for a dichotomized approach in chronic hepatitis (TE) | |
| Can be repeated easily but not validated for follow-up (TE) | |
| Disadvantages | Specific device requirement |
| Costly and limited access (MRI) | |
| Better performance for detecting cirrhosis than advanced fibrosis | |
| Lower applicability than serum markers (confounding factors for TE such as obesity, cholestasis, inflammation, congestion, food intake….) | |
| Gray zone for assessment of intermediate stage of fibrosis | |
- Abbreviations: MRI, magnetic resonance imaging; TE, transient elastography.
Noninvasive Techniques
Serum Biomarkers
Today, many of the current serum biomarker algorithms adopted into the clinical setting include a combination of peptides or proteins derived from fibrogenic cells, extracellular matrix remodeling, and simple biochemical tests that estimate disease severity. Most of the available noninvasive tests for fibrosis have been validated in hepatitis C, with relatively fewer studies in other important CLDs, with the algorithm dependent on the underlying liver disease. The AST-to-Platelet Ratio Index (APRI) and the FIB-4 are easily calculated indices.7, 8 Other patented marker panels (FibroTest, Fibrometer, Hepascore) are available (Table 2). The most widely used and validated are the APRI and the FibroTest, mainly in viral hepatitis C. A recent systematic review including 172 studies conducted in hepatitis C reported median areas under the receiver operating characteristic curve (AUROCs) of 0.79 and 0.86 for FibroTest and of 0.77 and 0.84 for APRI, for significant fibrosis and cirrhosis, respectively.9 Stepwise or sequential algorithms combining serum markers and imaging assessment appear to improve diagnostic accuracy for significant fibrosis and may reduce the need for biopsy. In the context of NAFLD, simple algorithms such as the NAFLD fibrosis score may differentiate advanced nonalcoholic steatohepatitis, but a substantial percentage of patients will have indeterminate values.10 This algorithm has now been recommended in practice guidelines as being clinically useful in identifying NAFLD patients with advanced disease. It is noteworthy that noninvasive techniques were initially developed for binary disease staging outcomes (for example METAVIR F0-1 versus F2-F4). Whether they are adapted to follow the dynamic changes of fibrogenesis or fibrosis regression is an important question that remains. Indeed, certain biomarkers such as granulocyte monocyte colony-stimulating factor, interleukin-12, interleukin-2, and matrix metalloproteinase 13 might be linked with changes in fibrosis stage, but this requires further validation.11
| Test | Variables | Liver Disease |
|---|---|---|
| APRI | AST, platelets | HCV |
| FIB-4 | Age, AST, ALT, platelets | HIV/HCV |
| FibroTest | Age, gender, bilirubin, GGT, α2M, haptoglobin, Apo-A1 | HCV |
| Hepascore | Age, gender, bilirubin, GGT, α2M, | HCV |
| Fibrometer | Age, AST, platelets, α2M, hyaluronan, prothrombin index, urea | HCV |
| Forns Index | Age, GGT, platelets, prothrombin, cholesterol | HCV |
| ELF Panel | Age, hyaluronan, TIMP-1, PNPIII | HCV |
| Fibrospectil | α2M, hyaluronan, TIMP-1 | HCV |
| NAFLD Fibrosis Score | Age, BMI, IFG, diabetes, AST/ALT, platelet count, albumin | NAFLD |
- Abbreviations: ALT, alanine aminotransferase; AST, asparate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transpeptidase; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NAFLD, nonalcoholic fatty liver disease; TIMP-1, tissue inhibitor of metalloproteinase-1.
Next-generation, high-throughput technologies have potential utility for providing more accurate blood biomarkers. Indeed, blood protein/peptide profiling may help to identify a novel biomarker for fibrosis, but the complex dynamic plasma protein range, issues regarding cost, variability in sample phenotype and depletion methods, technical reproducibility, and high-throughput capability still need to be addressed. MicroRNAs that regulate posttranscriptional gene expression appear to also regulate the fibrogenic cascade, and several have been proposed as potential markers of fibrosis in exosome-enriched fractionated serum.12 Epigenetic changes through DNA methylation also have a role in fibrosis and disease dynamics and might be potentially helpful. At this stage, clinical validation of such biomarkers that are detectable in blood or urine is still awaited. Long, noncoding RNA transcripts (>200 nucleotides) and genomic, metabolomic, and proteomic profiling of circulating extracellular vesicles (such as microvesicles, membrane particles, exosomes, or apoptotic bodies) implicated in disease pathogenesis and intercellular signaling represent novel approaches that hold promise for refined “liquid biopsy” biomarker development in the future.
Imaging Techniques
The introduction of liver stiffness measurement by using a variety of ultrasound (US)- or magnetic resonance (MR)-based techniques has been a major advance for the work-up of patients with CLD. Monodimensional US transient elastography (TE) (FibroScan; Echosens, Paris, France) is currently the most widely used and best-validated technique because it is a fast, safe, and reproducible procedure that can be performed at the bedside.13 TE more accurately detects cirrhosis (AUROC values, 0.87-0.98; correct classification ranging from 85% to 94%) than significant fibrosis (AUROC values, 0.75-0.93; correct classification from 57% to 90%).14 More recently, 2-dimensional elastography techniques embedded into conventional US machines (sonoelastography) have been introduced, such as Acoustic Radiation Force Impulse imaging (Acuson 2000 Virtual Touch Tissue Quantification; Siemens Healthcare, Erlangen, Germany) and ShearWave elastography (Aixplorer; Supersonic Imagine, Aix-en-Provence, France) (Table 3).15 Liver stiffness can be also measured by means of MR elastography using a modified phase-contrast method to image the propagation characteristics of the shear wave in the liver.16 It allows analysis of almost the entire liver, but MR elastography is thus far too costly and time-consuming to be used in routine practice.
| Technique | Imaging Modality | Advantages | Limitations |
|---|---|---|---|
| Monodimensional ultrasound transient elastography (Fibroscan) | Ultrasound | Easy to use | Ascites, obesity, congestion, cholestasis, acute inflammation, food intake |
| Highly standardized | |||
| Large clinical validation | |||
| Magnetic resonance elastography (MRE) | MRI | Penetration depth | Cost |
| 2D stiffness map | Availability limited | ||
| Lack of clinical validation | |||
| Acoustic radiation force impulse (ARFI) | Ultrasound | Image guidance | Penetration depth |
| Transient shear wave | High intensity ultrasound | ||
| Supersonic shear imaging (SSI) | Ultrasound | Image guidance | Penetration depth |
| Transient shear wave | High intensity ultrasound | ||
| Lack of clinical validation |
In addition to static evaluation, liver stiffness can be used to identify patients with cirrhosis who are at risk of disease progression. However, the diagnostic accuracy of TE is too low for identification of patients with esophageal varices in clinical practice.17 More recently, spleen stiffness has received much attention as a potential better predictor than liver stiffness, for analysis of portal hypertension. However, results remain conflicting. Several novel imaging technologies, such as perfusion, MR diffusion-weighted imaging, intravoxel incoherent motion, or acoustic structure quantification are being developed, but their use remains limited thus far to research settings.
Noninvasive methods for fibrosis assessment are now well implanted in clinical practice. However, we must keep in mind that fibrosis is only one of the many histopathologic features among a spectrum of liver injury observed in CLD, and these features are assessed together in a comprehensive manner only by liver biopsy. Therefore, liver biopsy will stay in a hepatologist's armamentarium for many years.




