Challenges in Diagnosis of Hepatocellular Carcinoma in Cirrhotic Liver: A Pathologist’s Perspective
Abbreviations
-
- CCA
-
- cholangiocarcinoma
-
- CK
-
- cytokeratin
-
- HCC
-
- hepatocellular carcinoma
-
- HGDN
-
- high-grade dysplastic nodule
-
- MA
-
- metastatic adenocarcinoma
This review focuses on the challenges in the pathological diagnosis of hepatocellular carcinoma (HCC) and explains some of the interpretative pitfalls particularly in very well-differentiated or poorly differentiated tumors. Although the diagnosis of HCC in cirrhosis often relies on radiological features, liver biopsy can enhance diagnostic specificity and enable molecular testing in an era of personalized medicine.
Typical Features of HCC
The pathological diagnosis of HCC is based on a combination of cytological features (nuclear enlargement, pleomorphism) and architectural atypia (widened trabeculae, pseudoglandular/acinar or solid growth pattern). Unpaired arteries without normal portal tracts are frequently seen (Fig. 1). Typical hepatocellular features, such as bile production, Mallory hyaline, and steatosis, can be observed. Histochemical stains like reticulin demonstrate loss or fragmentation of the reticulin network and help support a diagnosis of HCC, whereas demonstration of mucin with mucicarmine stain supports intrahepatic cholangiocarcinoma (CCA) or metastatic adenocarcinoma (MA) (Fig. 2).
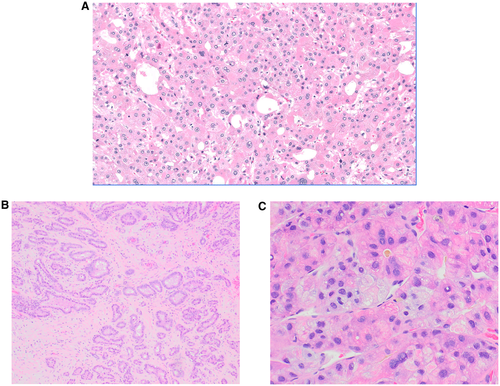
Immunohistochemistry is used to confirm hepatocellular differentiation; the most commonly used markers are arginase-1 (Arg-1), Hep Par 1, and glypican-3 (GPC-3). Most CCAs and MAs are positive for cytokeratin 7 (CK7), CK19, and/or MOC31. Albumin in situ hybridization helps in confirming hepatic origin because it is positive in both HCC and intrahepatic CCA, whereas most extrahepatic tumors are negative. Although these stains play a crucial role in confirming the diagnosis, the results have to be interpreted in the context of overall clinical setting and morphological features. For example, reticulin loss can be seen in nonneoplastic fatty liver; GPC-3 and, less commonly, Hep Par1 can be positive in CCA and MA; poorly differentiated HCC may be negative for Hep Par 1 and, less commonly, Arg-1; and HCC can be positive for CK7/CK19/MOC31 in 10% to 20% of cases (Table 1). These limitations necessitate the use of a panel of markers for diagnosis rather than rely on the results of a single marker.
| Stain | Utility | Limitations |
|---|---|---|
| Reticulin | Fragmented reticulin network in HCC | Similar findings can be seen in fatty liver |
| Mucicarmine | Demonstrate mucin in CCA or MA | Absence of mucin does not exclude CCA or MA |
| CD34 | Endothelial marker that highlights diffuse sinusoidal staining in HCC | Similar results can be seen in HGDN and benign lesions |
| Glutamine synthetase | Diffuse expression indicates β-catenin activation, supports HCC | Can be difficult to interpret; not specific for HCC |
| Arginase-1 | High sensitivity (>80%) and specificity (>90%) for HCC | Rarely positive in adenocarcinomas |
| Glypican-3 | High sensitivity (70%-80%) for poorly differentiated HCC | Not specific; can be seen in other malignant tumors and 5%-10% of CCAs |
| Hep Par 1 | High sensitivity (>80%) and specificity (>80%) | Less sensitive for poorly differentiated and scirrhous HCC; can be positive in gastroesophageal and lung adenocarcinomas |
| Albumin in situ hybridization | High sensitivity (>80%) | Also positive in CCA, acinar cell carcinoma, rarely in MA |
Challenges in Pathological Diagnosis
The most common diagnostic challenges are encountered at two ends of the differentiation spectrum of HCC (Table 2).
| High-Grade Dysplastic Nodule | Well-Differentiated HCC | Poorly Differentiated HCC | Intrahepatic CCA | |
|---|---|---|---|---|
| Morphological features | Small cell change | Small cell change | Solid or diffuse growth pattern | Glandular growth pattern in abundant fibrous stroma |
| Focally thick plates, few pseudoacini, few unpaired arterioles | Thick cell plates, pseudoglandular pattern, many unpaired arterioles | Marked cytological atypia | Mucin production | |
| Atypical features not enough for HCC | Invasion of portal tracts/adjacent liver | |||
| Reticulin | Intact or focal loss | Typically loss or fragmentation of reticulin framework | Loss or fragmentation of reticulin framework | No specific pattern |
| Mucicarmine | Negative | Negative | Negative | Can highlight mucin |
| CD34 | Sinusoidal staining at periphery, typically not diffuse | Diffuse sinusoidal staining | Diffuse sinusoidal staining | No specific pattern |
| Glutamine synthetase | Typically not diffuse | Can be diffuse | Can be diffuse | No specific pattern |
| Glypican-3 | Typically negative | Can be positive (<50%) | Often positive (~70%) | Rare positive (~5%) |
| Hep Par 1, Arginase-1 | Positive | Positive | Hep Par 50%-60%, Arginase 70%-80% | Typically negative |
| Albumin ISH | Positive | Positive | Positive | Positive |
| CK7, CK19, or MOC31 | Typically negative | Typically negative | Typically negative (positive in 10%-20%) | Typically positive |
- Abbreviation: ISH, in situ hybridization.
High-Grade Dysplastic Nodule Versus Well-Differentiated HCC
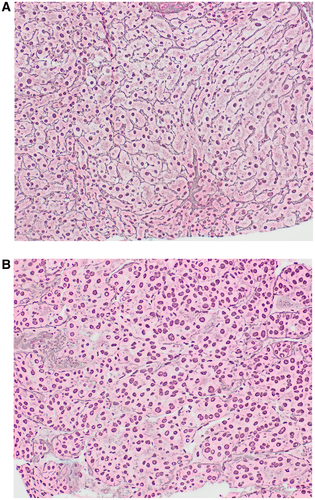
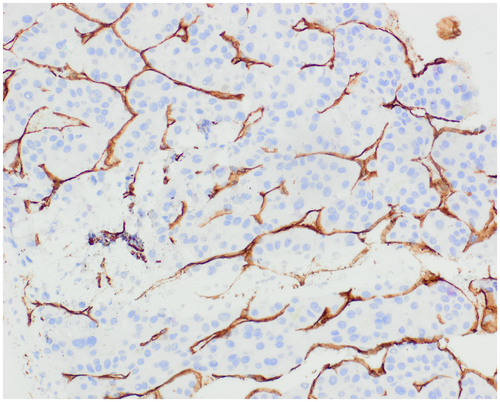
High-grade dysplastic nodule (HGDN) shares many features with HCC, including unpaired arterioles, small cell change, and pseudoacini, but these changes are focal.1, 2 Similarly, thick cell plates and reticulin loss are either absent or focal in HGDN and support HCC when multifocal. Invasion of portal tracts or adjacent liver is diagnostic of HCC. If the biopsy shows HGDN, the possibility of HCC is not excluded because progression to HCC may have involved only a portion of the nodule that may not have been sampled. Diffuse sinusoidal staining with CD34 is a result of capillarization and is more common in HCC than HGDN (Fig. 3). Demonstration of β-catenin activation by diffuse staining with glutamine synthetase or nuclear staining with β-catenin favors HCC (Fig. 4D). Similarly, positive results with GPC-3 and heat shock protein 70 (HSP70) favor HCC. It has been advocated that positive staining with two of these markers (GS, GPC-3, HSP70) is diagnostic of HCC; however, GPC-3 and HSP70 have low sensitivity in actual practice and are often not useful in this setting.3
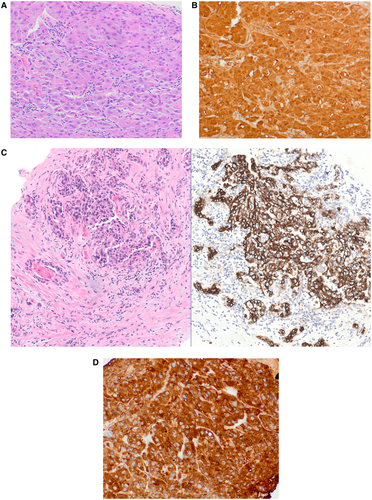
Poorly Differentiated HCC Versus Intrahepatic Cholangiocarcinoma
At the other end of the spectrum, poorly differentiated HCC may be difficult to distinguish from intrahepatic CCA. This distinction has a direct impact on treatment because of differences in chemotherapeutic regimens and possible denial of transplantation with CCA.4 Poorly differentiated HCC may not show typical morphological or immunohistochemical findings, while CK7, CK19, or MOC31 can be positive, leading to errors in diagnosis (Fig. 4A-C). Similarly, poorly differentiated CCA may lack gland formation and can mimic HCC. The combination of morphology, mucin stain, and immunohistochemistry allows a definite diagnosis in most cases. In some instances with overlapping results, next generation sequencing can help in the diagnosis. Mutations in TERT promoter and CTNNB1 (β-catenin) are characteristic of HCC, while mutations in IDH1/IDH2, PBRM1, and FGFR2 (fusion) point toward CCA. Similar to morphology and immunohistochemical findings, the mutational profile is not specific and the overall picture is taken into consideration for the final diagnosis.
Imaging Diagnosis of HCC in Cirrhotic Liver
- High specificity of diagnosis approaching 95% to 100%.8 The Liver Imaging Reporting and Data System (LI-RADS) is a classification system of reporting imaging findings of liver lesions, with LR-4 denoting probable HCC and LR-5 denoting definite HCC. Although the LR-5 category also has high specificity for HCC, some of these lesions have turned out to be other lesions, such as focal nodular hyperplasia or CCA on resection/explant.7 The sensitivity of HCC diagnosis on biopsy can vary based on the sampling.
- Several centers have used HCC differentiation as one of the criteria for making liver transplant decisions.9, 10 Poor differentiation is also an adverse prognostic criterion for HCC in explants.11
- Acquisition of tissue enables molecular testing for personalized therapy and clinical trials.12
- Significant bleeding and needle track seeding have been cited as adverse effects of liver biopsy, but are rare and do not affect disease course or overall survival.6
Conclusion
The pathological diagnosis of HCC requires integration, as well as awareness of pitfalls of morphological, histochemical, immunohistochemical, and molecular findings. Liver biopsy diagnosis of HCC has high specificity enabling appropriate therapy and also can play an important role in providing prognostic information and molecular data to guide treatment decisions, including transplant candidacy and personalized medicine.




