Electrochemical Conversion of N-Sulfinylamines to Sulfonimidoyl Fluorides
Fang-Ling Gao
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorXinglei He
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorBin Zhao
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorCorresponding Author
Yuqi Lin
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350108 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ke-Yin Ye
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350108 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorFang-Ling Gao
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorXinglei He
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorBin Zhao
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorCorresponding Author
Yuqi Lin
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350108 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ke-Yin Ye
Key Laboratory of Molecule Synthesis and Function Discovery (Fujian Province University), College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350108 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
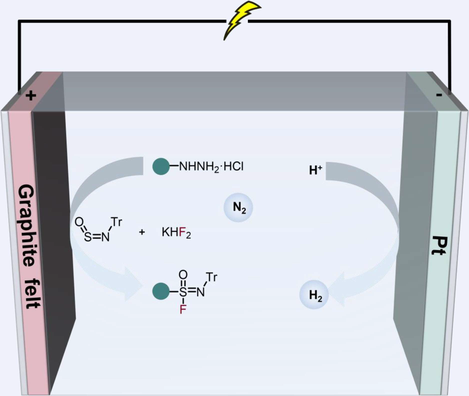
The invention of novel linkers is a long-lasting task in the area of the sulfur(VI) fluoride exchange reaction (SuFEx). Compared with the most frequently investigated sulfonyl fluorides, synthetic accessibility toward its mono-aza isostere, i.e., sulfonimidoyl fluorides is still limited. Herein, we report an electrochemical carbonfluorination of the readily available N-sulfinylamines to access various aryl and alkyl sulfonimidoyl fluorides. The transformation is characterized by the ready availability of starting materials, mild reaction conditions, and obviating metal catalysts and chemical oxidants.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202401088-sup-0001-supinfo.pdfPDF document, 6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Dong, J.; Krasnova, L.; Finn, M. G.; Sharpless, K. B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chem. Int. Ed. 2014, 53, 9430–9448; (b) Zhang, J.; Dong, J. Modular Click Chemistry Library: Searching for Better Functions. Chin. J. Chem. 2021, 39, 1025–1027; (c) Xu, L.; Dong, J. Click Chemistry: Evolving on the Fringe. Chin. J. Chem. 2020, 38, 414–419.
- 2(a) Barrow, A. S.; Smedley, C. J.; Zheng, Q.; Li, S.; Dong, J.; Moses, J. E. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 2019, 48, 4731–4758; (b) Jones, L. H.; Kelly, J. W. Structure-based design and analysis of SuFEx chemical probes. RSC Med.Chem. 2020, 11, 10–17; (c) Jin, P.; Shen, X. SulfoxFluor as a deoxyfluorination reagent, and beyond. J. Fluorine Chem. 2022, 261–262, 110029; (d) Thinnes, C. C.; Lohans, C. T.; Abboud, M. I.; Yeh, T.-L.; Tumber, A.; Nowak, R. P.; Attwood, M.; Cockman, M. E.; Oppermann, U.; Loenarz, C.; Schofield, C. J. Selective Inhibitors of a Human Prolyl Hydroxylase (OGFOD1) Involved in Ribosomal Decoding. Chem. Eur. J. 2019, 25, 2019–2024.
- 3 Zeng, D.; Deng, W.-P.; Jiang, X. Linkage Chemistry of S(VI) Fluorides. Chem. Eur. J. 2023, 29, e202300536.
- 4(a) Magre, M.; Ni, S.; Cornella, J. (Hetero)aryl-SVI Fluorides: Synthetic Development and Opportunities. Angew. Chem. Int. Ed. 2022, 61, e202200904; (b) Savoie, P. R.; Welch, J. T. Preparation and Utility of Organic Pentafluorosulfanyl-Containing Compounds. Chem. Rev. 2015, 115, 1130–1190.
- 5(a) Narayanan, A.; Jones, L. H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 2015, 6, 2650–2659; (b) Lin, L.; Pei, G.; Cao, Z.-Y.; Liao, S. Recent Advances in Developing Radical Methods for the Synthesis of Aliphatic Sulfonyl Fluorides. Eur. J. Org. Chem. 2024, 27, e202400279; (c) He, F.-S.; Li, Y.; Wu, J. Fluorosulfonyl radicals: new horizons for the synthesis of sulfonyl fluorides. Org. Chem. Front. 2022, 9, 5299–5305; (d) Lou, T. S.-B.; Willis, M. C. Sulfonyl fluorides as targets and substrates in the development of new synthetic methods. Nat. Rev. Chem. 2022, 6, 146–162; (e) Zeng, D.; Deng, W.-P.; Jiang, X. Advances in the construction of diverse SuFEx linkers. Nat. Sci. Rev. 2023, 10, nwad123; (f) Zheng, Y.; Lu, W.; Ma, T.; Huang, S. Recent advances in photochemical and electrochemical strategies for the synthesis of sulfonyl fluorides. Org. Chem. Front. 2024, 11, 217–235; (g) Zhang, H.; Xiao, W.; Wu, F.; Ma, X.; Liu, C. Recent progress in sulfonyl fluoride synthesis via the radical sulfur dioxide insertion and fluorination strategy. Sci. China Chem. 2024, DOI: https://doi.org/10.1007/s11426-024-2263-6.
- 6(a) Sirvent, J. A.; Lücking, U. Novel Pieces for the Emerging Picture of Sulfoximines in Drug Discovery: Synthesis and Evaluation of Sulfoximine Analogues of Marketed Drugs and Advanced Clinical Candidates. ChemMedChem 2017, 12, 487–501; (b) Frings, M.; Bolm, C.; Blum, A.; Gnamm, C. Sulfoximines from a Medicinal Chemist's Perspective: Physicochemical and in vitro Parameters Relevant for Drug Discovery. Eur. J. Med. Chem. 2017, 126, 225–245; (c) Lücking, U. Sulfoximines: A Neglected Opportunity in Medicinal Chemistry. Angew. Chem. Int. Ed. 2013, 52, 9399–9408; (d) Bizet, V.; Kowalczyk, R.; Bolm, C. Fluorinated sulfoximines: syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 2426–2438.
- 7(a) Garlyauskajte, R. Y.; Sereda, S. V.; Yagupolskii, L. M. The unusual reactivity of the mono- and bis- N-(trifluoromethylsulfonyl)-substituted azaanalogs of arenesulfonochlorides. Tetrahedron 1994, 50, 6891–6906;
(b) Yagupolskii, L. M.; Garlyauskajte, R. Y.; Kondratenko, N. V. N-(Trifluoromethylsulfonyl)arenesulfonimidoyl and N,N’-Bis(trifluoromethylsulfonyl)arenesulfonodiimidoyl Chlorides. Synthesis 1992, 1992, 749–750;
10.1055/s-1992-26215 Google Scholar(c) van Leusen, D.; van Leusen, A. M. Synthesis of arylsulfonimidoyl fluorides and their use in the preparation of (arylsulfonimidoyl)methyl isocyanides. Partial resolution of optically active S-phenyl-N-tosylsulfonimidoyl fluoride. Recl. Trav. Chim. Pays-Bas 1984, 103, 41–45.10.1002/recl.19841030201 Google Scholar
- 8(a) Liu, F.; Wang, H.; Li, S.; Bare, G. A. L.; Chen, X.; Wang, C.; Moses, J. E.; Wu, P.; Sharpless, K. B. Biocompatible SuFEx Click Chemistry: Thionyl Tetrafluoride (SOF4)-Derived Connective Hubs for Bioconjugation to DNA and Proteins. Angew. Chem. Int. Ed. 2019, 58, 8029–8033; (b) Gao, B.; Li, S.; Wu, P.; Moses, J. E.; Sharpless, K. B. SuFEx Chemistry of Thionyl Tetrafluoride (SOF4) with Organolithium Nucleophiles: Synthesis of Sulfonimidoyl Fluorides, Sulfoximines, Sulfonimidamides, and Sulfonimidates. Angew. Chem. Int. Ed. 2018, 57, 1939–1943; (c) Li, S.; Wu, P.; Moses, J. E.; Sharpless, K. B. Multidimensional SuFEx Click Chemistry: Sequential Sulfur(VI) Fluoride Exchange Connections of Diverse Modules Launched From An SOF4 Hub. Angew. Chem. Int. Ed. 2017, 56, 2903–2908.
- 9(a) Greed, S.; Symes, O.; Bull, J. A. Stereospecific reaction of sulfonimidoyl fluorides with Grignard reagents for the synthesis of enantioenriched sulfoximines. Chem. Commun. 2022, 58, 5387–5390; (b) Greed, S.; Briggs, E. L.; Idiris, F. I. M.; White, A. J. P.; Lücking, U.; Bull, J. A. Synthesis of Highly Enantioenriched Sulfonimidoyl Fluorides and Sulfonimidamides by Stereospecific Sulfur–Fluorine Exchange (SuFEx) Reaction. Chem. Eur. J. 2020, 26, 12533–12538.
- 10 Jiang, Y.-M.; Lin, Y.-Y.; Zhu, L.; Yu, Y.; Li, Y.; Lin, Y.; Ye, K.-Y. A General Electrochemical Synthesis of Sulfonimidoyl Fluorides, Azides, and Acetates. CCS Chem. 2024, 6, 2021–2030.
- 11 Davies, T. Q.; Willis, M. C. Rediscovering Sulfinylamines as Reagents for Organic Synthesis. Chem. Eur. J. 2021, 27, 8918–8927.
- 12 Davies, T. Q.; Hall, A.; Willis, M. C. One-Pot, Three-Component Sulfonimidamide Synthesis Exploiting the Sulfinylamine Reagent N-Sulfinyltritylamine, TrNSO. Angew. Chem. Int. Ed. 2017, 56, 14937–14941.
- 13 Lo, P. K. T.; Willis, M. C. Nickel(II)-Catalyzed Addition of Aryl and Heteroaryl Boroxines to the Sulfinylamine Reagent TrNSO: The Catalytic Synthesis of Sulfinamides, Sulfonimidamides, and Primary Sulfonamides. J. Am. Chem. Soc. 2021, 143, 15576–15581.
- 14 Andrews, J. A.; Kalepu, J.; Palmer, C. F.; Poole, D. L.; Christensen, K. E.; Willis, M. C. Photocatalytic Carboxylate to Sulfinamide Switching Delivers a Divergent Synthesis of Sulfonamides and Sulfonimidamides. J. Am. Chem. Soc. 2023, 145, 21623–21629.
- 15 Liu, Y.; Pan, Q.; Hu, X.; Guo, Y.; Chen, Q.-Y.; Liu, C. Rapid Access to N-Protected Sulfonimidoyl Fluorides: Divergent Synthesis of Sulfonamides and Sulfonimidamides. Org. Lett. 2021, 23, 3975–3980.
- 16(a) Firth, J. D.; Fairlamb, I. J. S. A Need for Caution in the Preparation and Application of Synthetically Versatile Aryl Diazonium Tetrafluoroborate Salts. Org. Lett. 2020, 22, 7057–7059; (b) Bremerich, M.; Conrads, C. M.; Langletz, T.; Bolm, C. Additions to N-Sulfinylamines as an Approach for the Metal-free Synthesis of Sulfonimidamides: O-Benzotriazolyl Sulfonimidates as Activated Intermediates. Angew. Chem. Int. Ed. 2019, 58, 19014–19020.
- 17(a) Kong, X.; Chen, Y.; Chen, X.; Ma, C.; Chen, M.; Wang, W.; Xu, Y.-Q.; Ni, S.-F.; Cao, Z.-Y. Organomediated electrochemical fluorosulfonylation of aryl triflates via selective C–O bond cleavage. Nat. Commun. 2023, 14, 6933; (b) Kong, X.; Chen, Y.; Liu, Q.; Wang, W.; Zhang, S.; Zhang, Q.; Chen, X.; Xu, Y.-Q.; Cao, Z.-Y. Selective Fluorosulfonylation of Thianthrenium Salts Enabled by Electrochemistry. Org. Lett. 2023, 25, 581-586; (c) Kong, X.; Liu, Q.; Chen, Y.; Wang, W.; Chen, H.-F.; Wang, W.; Zhang, S.; Chen, X.; Cao, Z.-Y. Direct electrochemical synthesis of arenesulfonyl fluorides from nitroarenes: a dramatic ionic liquid effect. Green Chem. 2024, 26, 3435–3440.
- 18 Woolven, H.; González-Rodríguez, C.; Marco, I.; Thompson, A. L.; Willis, M. C. DABCO-Bis(sulfur dioxide), DABSO, as a Convenient Source of Sulfur Dioxide for Organic Synthesis: Utility in Sulfonamide and Sulfamide Preparation. Org. Lett. 2011, 13, 4876–4878.
- 19(a) Ding, C.-L.; Xu, Q.; Wu, S.; Zhong, Y.; He, X.; Lin, Y.; Li, Y.; Ye, K.-Y. Current-Controlled Electrochemical Approach Toward Mono- and Trifluorinated Isoindolin-1-one Derivatives. Org. Lett. 2024, 26, 1645–1651; (b) Yu, Y.; Jiang, Y.; Wu, S.; Shi, Z.; Wu, J.; Yuan, Y.; Ye, K. Electrochemistry enabled selective vicinal fluorosulfenylation and fluorosulfoxidation of alkenes. Chin. Chem. Lett. 2022, 33, 2009–2014; (c) Shi, Z.; Wang, W.-Z.; Li, N.; Yuan, Y.; Ye, K.-Y. Electrochemical Dearomative Spirocyclization of N-Acyl Thiophene-2-sulfonamides. Org. Lett. 2022, 24, 6321–6325; (d) Yuan, G.-C.; Gao, F.-L.; Liu, K.-W.; Li, M.; Lin, Y.; Ye, K.-Y. Batch and Continuous-Flow Electrochemical Geminal Difluorination of Indeno[1,2-c]furans. Org. Lett. 2024, 26, 6059–6064.
- 20(a) Doobary, S.; Sedikides, A. T.; Caldora, H. P.; Poole, D. L.; Lennox, A. J. J. Electrochemical Vicinal Difluorination of Alkenes: Scalable and Amenable to Electron-Rich Substrates. Angew. Chem. Int. Ed. 2020, 59, 1155–1160; (b) Sawamura, T.; Kuribayashi, S.; Inagi, S.; Fuchigami, T. Use of Task-Specific Ionic Liquid for Selective Electrocatalytic Fluorination. Org. Lett. 2010, 12, 644–646; (c) Fuchigami, T.; Inagi, S. Recent Advances in Electrochemical Systems for Selective Fluorination of Organic Compounds. Acc. Chem. Res. 2020, 53, 322–334.
- 21(a) Yuan, Y.; Yang, J.; Lei, A. Recent advances in electrochemical oxidative cross-coupling with hydrogen evolution involving radicals. Chem. Soc. Rev. 2021, 50, 10058–10086; (b) Cheng, X.; Lei, A.; Mei, T.-S.; Xu, H.-C.; Xu, K.; Zeng, C. Recent Applications of Homogeneous Catalysis in Electrochemical Organic Synthesis. CCS Chem. 2022, 4, 1120–1152; (c) Novaes, L. F. T.; Liu, J.; Shen, Y.; Lu, L.; Meinhardt, J. M.; Lin, S. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 2021, 50, 7941–8002; (d) Wiebe, A.; Gieshoff, T.; Möhle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S. R. Electrifying Organic Synthesis. Angew. Chem. Int. Ed. 2018, 57, 5594–5619; (e) Meyer, T. H.; Choi, I.; Tian, C.; Ackermann, L. Powering the Future: How Can Electrochemistry Make a Difference in Organic Synthesis? Chem 2020, 6, 2484–2496; (f) Moeller, K. D. Using Physical Organic Chemistry to Shape the Course of Electrochemical Reactions. Chem. Rev. 2018, 118, 4817–4833; (g) Yan, M.; Kawamata, Y.; Baran, P. S. Synthetic Organic Electrochemical Methods Since 2000: on the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319; (h) Kwak, K.; Lee, D. Electrochemistry of Atomically Precise Metal Nanoclusters. Acc. Chem. Res. 2019, 52, 12–22.
- 22 Zhang, Z.-X.; Willis, M. C. Sulfondiimidamides as new functional groups for synthetic and medicinal chemistry. Chem 2022, 8, 1137–1146.




