Copper-Catalyzed Regioselective Cyanation/Diarylmethylation of Formamides: Construction of α-Cyano Functionalized Tetra-Substituted Olefins
Minjing Yuan
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
These authors contributed equally to this work.
Search for more papers by this authorZikang Li
Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, China
These authors contributed equally to this work.
Search for more papers by this authorWeifeng Xu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Biquan Xiong
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorYu Liu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Min Liu
School of Chemistry and Materials Engineering, Huizhou University, Huizhou, Guangdong, 516007 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorKe-Wen Tang
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Longzhi Zhu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorMinjing Yuan
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
These authors contributed equally to this work.
Search for more papers by this authorZikang Li
Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, China
These authors contributed equally to this work.
Search for more papers by this authorWeifeng Xu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Biquan Xiong
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorYu Liu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Min Liu
School of Chemistry and Materials Engineering, Huizhou University, Huizhou, Guangdong, 516007 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorKe-Wen Tang
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Longzhi Zhu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
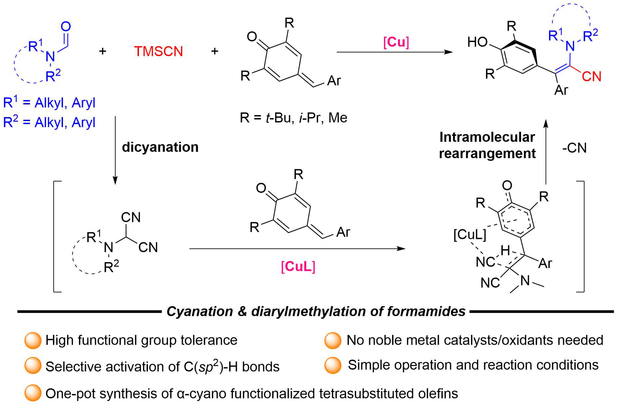
A copper-catalyzed cyanation/diarylmethylation of formamides has been developed for the synthesis of α-cyano functionalized tetra-substituted olefins by utilizing para-quinone methides (p-QMs) and trimethylcyanosilane as functionalization sources. Various kinds of p-QMs and formamides are well tolerated, delivering the desired products with 72%—94% yields, demonstrating broad functional group tolerance. Notably, the reaction does not require noble metals and proceeds regioselectively under mild conditions. Based on step-by-step control experiments, Hammett studies and DFT calculation, a plausible mechanism is proposed.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400911-sup-0001-supinfo.pdfPDF document, 6.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Zhang, J.-H.; Xu, H.; Tang, X.; Dang, Y.; Zhang, F.-G.; Ma, J.-A. Highly Enantio- and Diastereoselective Hydrogenation of Cyclic Tetra-substituted β-Enamido Phosphorus Derivatives. Angew. Chem. Int. Ed. 2023, 62, e202305315;
(b) Bao, W.; Chen, Y. H.; Liu, Y. W.; Xiang, S. H.; Tan, B. Atroposelective Synthesis of 2-Arylindoles via Chiral Phosphoric Acid-Catalyzed Direct Amination of Indoles. Chin. J. Chem. 2024, 42, 731–735;
(c) Cai, Z. N.; Feng, X. X.; Zhang, Y.; Lu, C. C.; Han, Y. P.; Zhao, J. Transition-Metal-Free Catalyzed Dehydrative Coupling of Quinoline and Isoquinoline N-Oxides with Propargylic Alcohols. Chin. J. Chem. 2021, 40, 71–78;
10.1002/cjoc.202100687 Google Scholar(d) Xing, W.-L.; Wang, J. X.; Fu, M. C.; Fu, Y. Efficient Decarboxylative/Defluorinative Alkylation for the Synthesis of gem-Difluoroalkenes through an SN2’-Type Route. Chin. J. Chem. 2021, 40, 323–328.
- 2(a) Cowden, A. M.; Whittock, A. L.; Holt, E. L.; Stavros, V. G.; Wills, M. Synthesis and characterisation of novel composite sunscreens containing both avobenzone and octocrylene motifs. RSC Adv. 2023, 13, 17017–17027; (b) Chen, D.; Long, Z.; Zhong, C.; Chen, L.; Dang, Y.; Hu, J.-J.; Lou, X.; Xia, F. Highly Efficient Near-Infrared Photosensitizers with Aggregation-Induced Emission Characteristics: Rational Molecular Design and Photodynamic Cancer Cell Ablation. ACS Appl. Bio Mater. 2021, 4, 5231–5239; (c) de Groot, A. C.; Roberts, D. W. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis 2014, 70, 193–204; (d) Zhang, L.-H.; Wu, L.; Raymon, H. K.; Chen, R. S.; Corral, L.; Shirley, M. A.; Krishna Narla, R.; Gamez, J.; Muller, G. W.; Stirling, D. I.; Bartlett, J. B.; Schafer, P. H.; Payvandi, F. The Synthetic Compound CC-5079 Is a Potent Inhibitor of Tubulin Polymerization and Tumor Necrosis Factor-α Production with Antitumor Activity. Cancer Res. 2006, 66, 951–959.
- 3 Ogilvie, A. B. F. a. W. W. Stereocontrolled Synthesis of Tetrasubstituted Olefins. Chem. Rev. 2007, 107, 4698–4745.
- 4(a) Maryanoff, B. E.; Reitz, A. B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 1989, 89, 863–927; (b) Edmonds, M.; Abell, A. In Modern Carbonyl Olefination, Wiley-VCH, 2003, ch. 1, pp. 1–17; (c) Li, B. X.; Le, D. N.; Mack, K. A.; McClory, A.; Lim, N.-K.; Cravillion, T.; Savage, S.; Han, C.; Collum, D. B.; Zhang, H.; Gosselin, F. Highly Stereoselective Synthesis of Tetrasubstituted Acyclic All-Carbon Olefins via Enol Tosylation and Suzuki-Miyaura Coupling. J. Am. Chem. Soc. 2017, 139, 10777–10783.
- 5(a) Olivares, A. M.; Weix, D. J. Multimetallic Ni- and Pd-Catalyzed Cross-Electrophile Coupling To Form Highly Substituted 1,3-Dienes. J. Am. Chem. Soc. 2018, 140, 2446–2449; (b) Kraft, S.; Ryan, K.; Kargbo, R. B. Recent Advances in Asymmetric Hydrogenation of Tetrasubstituted Olefins. J. Am. Chem. Soc. 2017, 139, 11630–11641.
- 6(a) Arora, R.; Rodríguez, J. F.; Whyte, A.; Lautens, M. Accessing Unsymmetrically Linked Heterocycles through Stereoselective Palladium-Catalyzed Domino Cyclization. Angew. Chem. Int. Ed. 2022, 61, e202112288; (b) Hewitt, K. A.; Xie, P.-P.; Thane, T. A.; Hirbawi, N.; Zhang, S.-Q.; Matus, A. C.; Lucas, E. L.; Hong, X.; Jarvo, E. R. Nickel-Catalyzed Domino Cross-Electrophile Coupling Dicarbofunctionalization Reaction To Afford Vinylcyclopropanes. ACS Catal. 2021, 11, 14369–14380; (c) Lv, W.; Liu, S.; Chen, Y.; Wen, S.; Lan, Y.; Cheng, G. Palladium-Catalyzed Intermolecular Trans-Selective Carbofunctionalization of Internal Alkynes to Highly Functionalized Alkenes. ACS Catal. 2020, 10, 10516–10522.
- 7 Li, Y.; Shao, Q.; He, H.; Zhu, C.; Xue, X.-S.; Xie, J. Highly selective synthesis of all-carbon tetrasubstituted alkenes by deoxygenative alkenylation of carboxylic acids. Nat. Commun. 2022, 13, 1–7.
- 8(a) Chen, J.; Chen, S.; Xu, X.; Tang, Z.; Au, C.-T.; Qiu, R. Nickel- Catalyzed Regioselective Cleavage of Csp2–S Bonds: Method for the Synthesis of Tri- and Tetrasubstituted Alkenes. J. Org. Chem. 2016, 81, 3246–3255; (b) Li, J.; Ren, Q.; Cheng, X.; Karaghiosoff, K.; Knochel, P. Chromium(II)-Catalyzed Diastereoselective and Chemoselective Csp2–Csp3 Cross-Couplings Using Organomagnesium Reagents. J. Am. Chem. Soc. 2019, 141, 18127–18135; (c) Li, B.-J.; Xu, L.; Wu, Z.-H.; Guan, B.-T.; Sun, C.-L.; Wang, B.-Q.; Shi, Z.-J. Cross-Coupling of Alkenyl/Aryl Carboxylates with Grignard Reagent via Fe-Catalyzed C-O Bond Activation. J. Am. Chem. Soc. 2009, 131, 14656–14657; (d) Gärtner, D.; Stein, A. L.; Grupe, S.; Arp, J.; Jacobi von Wangelin, A. Iron-Catalyzed Cross-Coupling of Alkenyl Acetates. Angew. Chem. Int. Ed. 2015, 54, 10545–10549; (e) Rivera, A. C. P.; Still, R.; Frantz, D. E. Iron-Catalyzed Stereoselective Cross-Coupling Reactions of Stereodefined Enol Carbamates with Grignard Reagents. Angew. Chem. Int. Ed. 2016, 55, 6689–6693.
- 9 Li, J.; Knochel, P. Cobalt-Catalyzed Cross-Couplings between Alkenyl Acetates and Aryl or Alkenyl Zinc Pivalates. Angew. Chem. Int. Ed. 2018, 57, 11436–11440.
- 10 Li, W.; Yu, S.; Li, J.; Zhao, Y. Nickel-Catalyzed Allylmethylation of Alkynes with Allylic Alcohols and AlMe3: Facile Access to Skipped Dienes and Trienes. Angew. Chem. Int. Ed. 2020, 59, 14404–14408.
- 11(a) Wienecke, P.; Arndt, H.-D. Direct C–H Cyanation by ICN Formed In Situ: Nannozinone B. Org. Lett. 2023, 25, 1188–1191; (b) Mou, X.-Q.; Xu, Z.-L.; Xu, L.; Wang, S.-H.; Zhang, B.-H.; Zhang, D.; Wang, J.; Liu, W.-T.; Bao, W. The Synthesis of Multisubstituted Pyrroles via a Copper-Catalyzed Tandem Three-Component Reaction. Org. Lett. 2016, 18, 4032–4035; (c) Wang, X.; Studer, A. Metal-Free Direct C−H Cyanation of Alkenes. Angew. Chem. Int. Ed. 2018, 57, 11792–11796; (d) Jiang, L.-F.; Ren, B.-T.; Li, B.; Zhang, G.-Y.; Peng, Y.; Guan, Z.-Y.; Deng, Q.-H. Nucleophilic Substitution of gem-Difluoroalkenes with TMSNu Promoted by Catalytic Amounts of Cs2CO3. J. Org. Chem. 2019, 84, 6557–6564.
- 12(a) Wang, J.-Y.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Recent developments in 1,6-addition reactions of para-quinone methides (p-QMs). Org. Chem. Front. 2020, 7, 1743–1778; (b) Lima, C. G. S.; Pauli, F. P.; Costa, D. C. S.; Souza, A. S. d.; Forezi, L. S. M.; Ferreira, V. F.; Silva, F. d. C. d. para-Quinone Methides as Acceptors in 1,6-Nucleophilic Conjugate Addition Reactions for the Synthesis of Structurally Diverse Molecules. Eur. J. Org. Chem. 2020, 2020, 2650–2692; (c) Zhang, Z.-P.; Dong, N.; Li, X. Bismuth-catalyzed allylation of para-quinone methides. Chem. Commun. 2017, 53, 1301–1304; (d) Zi, P.-Z.; Liu, X.-B.; Zhao, Q.-H.; He, M.; Huang, Y. 1,6-Conjugate addition of para-quinone methides using gem-diborylcarbanions: Practical access to gem-diborylalkanes bearing vicinal tertiary/quaternary stereocenters. Green Synth. Catal. 2024, 5, 68–72; (e) Li, S.; Liu, Y.; Huang, B.; Zhou, T.; Tao, H.; Xiao, Y.; Liu, L.; Zhang, J. Phosphine-Catalyzed Asymmetric Intermolecular Cross-Vinylogous Rauhut–Currier Reactions of Vinyl Ketones with para-Quinone Methides. ACS Catal. 2017, 7, 2805–2809.
- 13(a) Singh, G.; Pandey, R.; Pankhade, Y. A.; Fatma, S.; Anand, R. V. Construction of Oxygen- and Nitrogen-based Heterocycles from p-Quinone Methides. Chem. Rec. 2021, 21, 4150–4173; (b) Liu, X.; Ren, Y.; Zhu, L.; Li, T.; Xu, W.; Liu, Y.; Tang, K.-W.; Xiong, B. Recent advances in the cyclization of para-quinone methides. Tetrahedron 2023, 148, 133655; (c) Maestro, A.; Zurro, M. Phosphine-catalysed transformations of ortho- and para-quinone methides. Org. Biomol. Chem. 2023, 21, 8244–8258; (d) Ma, C.; Huang, Y.; Zhao, Y. Stereoselective 1,6-Conjugate Addition/Annulation of para-Quinone Methides with Vinyl Epoxides/Cyclopropanes. ACS Catal. 2016, 6, 6408–6412; (e) Chen, K.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Metal-free synthesis of triarylated (Z)-nitrones via H2O-mediated 1,3-dipolar transfer under aerobic conditions. Green Chem. 2019, 21, 675–683.
- 14(a) Fan, L.; Zhu, L.; Shang, W.; Yin, M.; Xiong, B.; Xie, W.; Cao, F.; Liu, Y.; Chen, Y.; Qiu, R. Cascade Cyclization/Amination of para-Quinone Methides with β-Ketodinitriles: Synthesis of Polysubstituted Furans. Adv. Synth. Catal. 2024, 366, 2101–2108; (b) Zhu, L.; Ren, Y.; Liu, X.; Xu, S.; Li, T.; Xu, W.; Li, Z.; Liu, Y.; Xiong, B. Catalyst- and Additive-free, Regioselective 1,6-Hydroarylation of para-Quinone Methides with Anilines in HFIP. Chem. Asian J. 2023, 18, e202300792; (c) Xiong, B.; Xu, S.; Xu, W.; Liu, Y.; Zhang, L.; Tang, K.-W.; Yin, S.-F.; Wong, W.-Y. Silver-catalyzed regioselective 1,6-hydroarylation of para-quinone methides with anilines and phenols. Org. Chem. Front. 2022, 9, 3807–3817; (d) Xu, S.; Xie, J.; Liu, Y.; Xu, W.; Tang, K.-W.; Xiong, B.; Wong, W.-Y. Silver-Catalyzed Regioselective Phosphorylation of para-Quinone Methides with P(III)-Nucleophiles. J. Org. Chem. 2021, 86, 14983–15003.
- 15 Rathod, J.; Sharma, B.; Mali, P.; Kumar, P. Tf2NH-Catalyzed 1,6-Conjugate Addition of Vinyl Azides with p-Quinone Methides: A Mild and Efficient Method for the Synthesis of β-Bis-Arylamides. Synthesis 2017, 49, 5224–5230.
- 16(a) Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305; (b) Zheng, J.; Xu, X.; Truhlar, D. G. Minimally augmented Karlsruhe basis sets. Theor. Chem. Acc. 2010, 128, 295–305.




