Catalyst-Free, Radical Tri- and Difluoromethylation of Isocyanides and N-Arylacrylamides Using Rotating Magnetic Field and Metal Rods
Xuliang Han
School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
Search for more papers by this authorHaodong Liu
School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
Search for more papers by this authorXiaomei Feng
School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
Search for more papers by this authorFuchao Jia
School of Physics and Optoelelctronic Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
Search for more papers by this authorZengdian Zhao
School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
Search for more papers by this authorCorresponding Author
Xinjin Li
School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
E-mail: [email protected]Search for more papers by this authorXuliang Han
School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
Search for more papers by this authorHaodong Liu
School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
Search for more papers by this authorXiaomei Feng
School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
Search for more papers by this authorFuchao Jia
School of Physics and Optoelelctronic Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
Search for more papers by this authorZengdian Zhao
School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
Search for more papers by this authorCorresponding Author
Xinjin Li
School of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255000 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
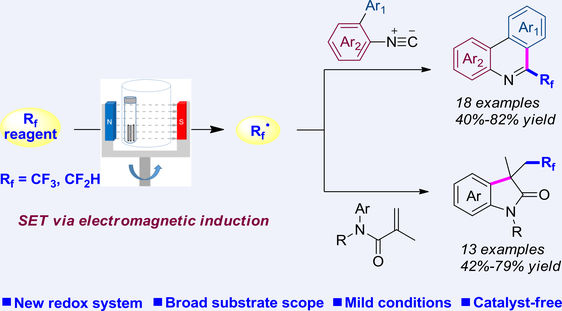
The pursuit of sustainable and environmentally benign synthetic methods continues to challenge organic chemists. Herein, we introduce a magnetoredox system for tri- and difluoromethylation of isocyanides and N-arylacrylamides using a rotating magnetic field and steel rods. This magnetoredox approach enables facile synthesis of functionalized phenanthridines and oxindoles without the need for catalysts and additives under mild conditions. Such a system potentially represents an attractive strategy for selective formation of bonds through multifaceted regulation of magnetic intensity, rotating frequency, and rod size.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400854-sup-0001-supinfo.pdfPDF document, 14.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Narayanam, J. M. R.; Stephenson, C. R. J. Visible Light Photoredox Catalysis: Applications in Organic Synthesis. Chem. Soc. Rev. 2011, 40, 102–113; (b) Prier, C. K., Rankic, D. A.; MacMillan, D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363; (c) Yu, X.-Y., Chen, J.-R.; Xiao, W.-J. Visible Light-Driven Radical-Mediated C–C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561; (d) Bellotti, P.; Huang, H.-M.; Faber, T.; Glorius, F. Photocatalytic Late-Stage C–H Functionalization. Chem. Rev. 2024, 123, 4237–4352.
- 2(a) Kubota, K.; Pang, Y.; Miura, A.; Ito, H. Redox Reactions of Small Organic Molecules Using Ball Milling and Piezoelectric Materials. Science 2019, 366, 1500–1504; (b) Schumacher, C., Hernández, J. G.; Bolm, C. Electro-Mechanochemical Atom Transfer Radical Cyclizations Using Piezoelectric BaTiO3. Angew. Chem. Int. Ed. 2020, 59, 16357–16360; (c) Pang, Y., Lee, J. W., Kubota, K.; Ito, H. Solid-State Radical C−H Trifluoromethylation Reactions Using Ball Milling and Piezoelectric Materials. Angew. Chem. Int. Ed. 2020, 59, 22570–22576; (d) Lv, H.; Xu, X.; Li, J.; Huang, X.; Fang, G.; Zheng, L. Mechanochemical Divergent Syntheses of Oxindoles and α-Arylacylamides via Controllable Construction of C−C and C−N Bonds by Copper and Piezoelectric Materials. Angew. Chem. Int. Ed. 2022, 61, e202206420; (e) Wang, X.; Zhang, X.; Xue, L.; Wang, Q.; You, F.; Dai, L.; Wu, J.; Kramer, S.; Lian, Z. Mechanochemical Synthesis of Aryl Fluorides by Using Ball Milling and A Piezoelectric Material as the Redox Catalyst. Angew. Chem. Int. Ed. 2023, 62, e202307054; (f) Seo, T.; Kubota, K.; Ito, H. Dual Nickel(II)/Mechanoredox Catalysis: Mechanical-Force- Driven Aryl-Amination Reactions Using Ball Milling and Piezoelectric Materials. Angew. Chem. Int. Ed. 2023, 62, e202311531.
- 3 Liu, H.; Han, X.; Feng, X.; Zhang, L.; Sun, F.; Jia, F.; Zhao, Z.; Liu, H.; Li, X. Redox Reactions of Organic Molecules Using Rotating Magnetic Field and Metal Rods. J. Am. Chem. Soc. 2024, 146, 18143–18150.
- 4(a) Bégué, J.-P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine, Wiley, Hoboken, 2008, pp. 1−22;
10.1002/9780470281895 Google Scholar(b) Purser, S., Moore, P. R., Swallow, S.; Gouverneur, V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320−330; (c) Ni, C.; Hu, J. The Unique Fluorine Effects in Organic Reactions: Recent Facts and Insights into Fluoroalkylations. Chem. Soc. Rev. 2016, 45, 5441−5454.
- 5(a) Charpentier, J., Früh, N.; Togni, A. Electrophilic Trifluoromethylation by Use of Hypervalent Iodine Reagents. Chem. Rev. 2015, 115, 650–682;
(b) Ni, C.; Hu, M.; Hu, J. Good Partnership between Sulfur and Fluorine: Sulfur-Based Fluorination and Fluoroalkylation Reagents for Organic Synthesis. Chem. Rev. 2015, 115, 765−825;
(c) Li, X.; Liu, J.; Li, X.; Liu, H.; Liu, H.; Li, Y.; Liu, Y.; Dong, Y. Recent Advance in the Synthesis of (1,1-Difluoroethyl)arenes. J. Fluorine Chem. 2018, 216, 102−106;
(d) Xiao, H.-W., Zhang, Z.-Z., Fang, Y.-W., Zhu, L.; Li, C.-Z. Radical Trifluoromethylation. Chem. Soc. Rev. 2021, 50, 6308–63073;
(e) Lin, D.; Coe, M.; Krishnamurti, V.; Ispizua-Rodriguez, X.; Prakash, G. K. S. Recent Advances in Visible Light-Mediated Radical Fluoro-Alkylation, -Alkoxylation, -Alkylthiolation, -Alkylselenolation, and -Alkylamination. Chem. Rec. 2024, 23, e202300104.
10.1002/tcr.202300104 Google Scholar
- 6(a) Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; Pozo, C. D.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001−2011). Chem. Rev. 2014, 114, 2432−2506; (b) Ogawa, Y., Tokunaga, E., Kobayashi, O., Hirai, K.; Shibata, N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467–101519.
- 7(a) Koike, T.; Akita, M. New Horizons of Photocatalytic Fluoromethylative Difunctionalization of Alkenes. Chem 2018, 4, 409–437; (b) Ouyang, Y.; Qing, F.-L. Photoredox Catalyzed Radical Fluoroalkylation with Non-Classical Fluorinated Reagents. J. Org. Chem. 2024, 89, 2815–2824, and references therein.
- 8(a) Sap, J. B. I.; Meyer, C. F.; Straathof, N. J. W.; Iwumene, N.; am Ende, C. W.; Traban, A. A.; Gouverneur, V. Late-Stage Difluoromethylation: Concepts, Developments and Perspective. Chem. Soc. Rev. 2021, 50, 8214–8247; (b) Feng, J.; Jia, X.; Zhang, S.; Lu, K.; Cahard, D. State of Knowledge in Photoredox-Catalysed Direct Difluoromethylation. Org. Chem. Front. 2022, 9, 3598–3623, and references therein.
- 9 Jia, H.; Häring, A. P.; Berger, F.; Zhang, L.; Ritter, T. Trifluoromethyl Thianthrenium Triflate: A Readily Available Trifluoromethylating Reagent with Formal CF3+, CF3•, and CF3– Reactivity. J. Am. Chem. Soc. 2021, 143, 7623−7628.
- 10(a) Zhang, B.; Mück-Lichtenfeld, C.; Daniliuc, C. G.; Studer, A. 6-Trifluoromethyl-Phenanthridines Through Radical Trifluoromethylation of Isonitriles. Angew. Chem. Int. Ed. 2013, 52, 10792−10795; (b) Rong, J.; Deng, L.; Tan, P.; Ni, C.; Gu, Y.; Hu, J. Radical Fluoroalkylation of Isocyanides with Fluorinated Sulfones by Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2016, 55, 2743−2747.
- 11(a) Wang, Y., Wang, J., Li, G.-X., He, G.; Chen, G. Halogen-Bond-Promoted Photoactivation of Perfluoroalkyl Iodides: A Photochemical Protocol for Perfluoroalkylation Reactions. Org. Lett. 2017, 19, 1442–1445;
(b) Li, J., Caiuby, C. A. D., Paixão, M. W.; Li, C.-J. Synthesis of 6-Trifluoromethylphenanthridines through Radical Trifluoromethylation of Isocyanides with Sodium Triflinate under Visible Light. Eur. J. Org. Chem. 2018, 2498–2503;
10.1002/ejoc.201701487 Google Scholar(c) Liu, S.; Pan, W.; Wu, S.; Bu, X.; Xin, S.; Yu, J.; Xu, H.; Yang, X. Visible-Light-Induced Tandem Radical Addition-Cyclization of 2-Aryl Phenyl Isocyanides Catalysed by Recyclable Covalent Organic Frameworks. Green Chem. 2019, 21, 2905−2910; (d) Wang, Y.; Cao, Z.; He, Q.; Huang, X.; Liu, J.; Neumann, H.; Chen, G.; Beller, M. Activation of Perfluoroalkyl Iodides by Anions: Extending the Scope of Halogen Bond Activation to C(Sp3)-H Amidation, C(Sp2)-H Iodination, and Perfluoroalkylation Reactions. Chem. Sci. 2023, 14, 1732−1741.
- 12(a) Zhang, J.; Coote, M. L.; Ciampi, S. Electrostatics and Electrochemistry: Mechanism and Scope of Charge-Transfer Reactions on The Surface of Tribocharged Insulators. J. Am. Chem. Soc. 2021, 143, 3019−3032; (b) Yoo, D.; Jang, S.; Cho, S.; Choi, D.; Kim, D. S. A Liquid Triboelectric Series. Adv. Mater. 2023, 35, 2300699.
- 13(a) Xu, P.; Xie, J.; Xue, Q.; Pan, C.; Cheng, Y.; Zhu, C. Visible-Light-Induced Trifluoromethylation of N-Aryl Acrylamides: A Convenient and Effective Method to Synthesize CF3-Containing Oxindoles Bearing a Quaternary Carbon Center. Chem. Eur. J. 2013, 19, 14039–14042; (b) Tang, X.-J.; Thomoson, C. S.; Dolbier Jr., W. R. Photoredox-Catalyzed Tandem Radical Cyclization of N-Arylacrylamides: General Methods to Construct Fluorinated 3,3-Disubstituted 2-Oxindoles Using Fluoroalkylsulfonyl Chlorides. Org. Lett. 2014, 16, 4594–4597.
- 14(a) Studer, A.; Curran, D. P. The Electron Is a Catalyst. Nat. Chem. 2014, 6, 765−773; (b) Cismesia, M. A.; Yoon, T. P. Characterizing Chain Processes in Visible Light Photoredox Catalysis. Chem. Sci. 2015, 6, 5426−5434; (c) Syroeshkin, M. A.; Kuriakose, F.; Saverina, E. A.; Timofeeva, V. A.; Egorov, M. P.; Alabugin, I. V. Upconversion of Reductants. Angew. Chem. Int. Ed. 2019, 58, 5532−5550.




