Cycloaddition Reactions of Epoxides and CO2 Catalyzed by Bifunctional Rare-Earth Metal Complexes Bearing Amino-Bridged Tris(phenolato) Ligands
Yongjie Chen
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Dushu Lake Campus, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorYanwei Wang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Dushu Lake Campus, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorJun Nong
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Dushu Lake Campus, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Dan Yuan
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Dushu Lake Campus, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Yingming Yao
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Dushu Lake Campus, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected], [email protected]Search for more papers by this authorYongjie Chen
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Dushu Lake Campus, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorYanwei Wang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Dushu Lake Campus, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorJun Nong
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Dushu Lake Campus, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Dan Yuan
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Dushu Lake Campus, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Yingming Yao
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Dushu Lake Campus, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected], [email protected]Search for more papers by this authorComprehensive Summary
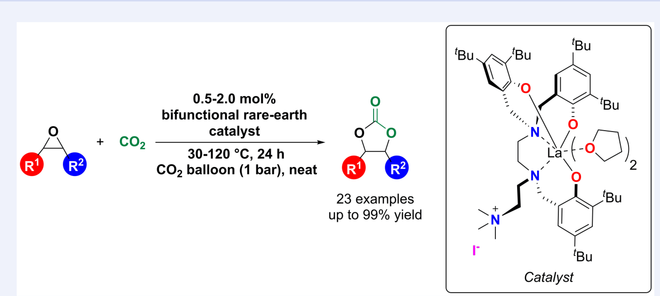
Eight zwitterionic rare earth metal complexes stabilized by amino-bridged tris(phenolato) ligands bearing quaternary ammonium side-arms were synthesized and characterized. These complexes were used as single-component catalysts for the cycloaddition of CO2 and epoxides, and their catalytic activities are obviously higher than those of their binary analogues. Further studies revealed that the halide anions (Cl–, Br–, I–) and the metal complexes influenced the catalytic activity, and the lanthanum complex bearing iodide anion showed the highest catalytic activity for this addition reaction. A variety of mono-substituted epoxides were converted to cyclic carbonates in good to excellent yields (55%—99%) with high selectivity (> 99%) at 30 °C and 1 bar CO2, whereas internal epoxides required higher both reaction temperatures (60—120 °C) and catalyst loading (2 mol%) for high yields. The catalyst was recyclable for four times without noticeable loss of catalytic activity. Based on the results of kinetic studies and in situ IR reactions, a plausible reaction mechanism was proposed.
Supporting Information
| Filename | Description |
|---|---|
| cjoc_202300753_sm_suppl.pdfPDF document, 8.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using Carbon Dioxide as a Building Block in Organic Synthesis. Nat. Commun. 2015, 6, 5933; (b) Artz, J.; Muller, T. E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: an Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504.
- 2(a) Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E. A.; Fuss, S.; Mac Dowell, N.; Minx, J. C.; Smith, P.; Williams, C. K. The Technological and Economic Prospects for CO2 Utilization and Removal. Nature 2019, 575, 87–97; (b) Ye, J.-H.; Ju, T.; Huang, H.; Liao, L.-L.; Yu, D.-G. Radical Carboxylative Cyclizations and Carboxylations with CO2. Acc. Chem. Res. 2021, 54, 2518–2531; (c) Wang, Y.; Tang, S.; Yang, G.; Wang, S.; Ma, D.; Qiu, Y. Electrocarboxylation of Aryl Epoxides with CO2 for the Facile and Selective Synthesis of β-Hydroxy Acids. Angew. Chem. Int. Ed. 2022, 61, e202207746; (d) Zhang, K.; Ren, B.-H.; Liu, X.-F.; Wang, L.-L.; Zhang, M.; Ren, W.-M.; Lu, X.-B.; Zhang, W.-Z. Direct and Selective Electrocarboxylation of Styrene Oxides with CO2 for Accessing β-Hydroxy Acids. Angew. Chem. Int. Ed. 2022, 61, e202207660; (e) Gao, T.-Y.; Mo, X.-Y.; Zhang, S.-R.; Jiang, Y.-X.; Luo, S.-P.; Ye, J.-H.; Yu, D.-G. Visible-light photoredox-catalyzed carboxylation of aryl epoxides with CO2. Chin. Chem. Lett. 2023, 109364.
- 3(a) Trost, B. M. The Atom Economy—a Search for Synthetic Efficiency. Science 1991, 254, 1471–1477; (b) Bhat, G. A.; Darensbourg, D. J. Progress in the Catalytic Reactions of CO2 and Epoxides to Selectively Provide Cyclic or Polymeric Carbonates. Green Chem. 2022, 24, 5007–5034.
- 4 Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618.
- 5(a) Schaffner, B.; Schaffner, F.; Verevkin, S. P.; Borner, A. Organic Carbonates as Solvents in Synthesis and Catalysis. Chem. Rev. 2010, 110, 4554–4581; (b) Sathish, M.; Thaikaivelan, P.; Rao, J. R. Application of GSK's Model in Leather Making: Quantification of the Environmental Efficiency of a Green Solvent Based Deliming Process. ACS Sustainable Chem. Eng. 2022, 10, 4943–4953.
- 6(a) Ngassam Tounzoua, C.; Grignard, B.; Detrembleur, C. Exovinylene Cyclic Carbonates: Multifaceted CO2-based Building Blocks for Modern Chemistry and Polymer Science. Angew. Chem. Int. Ed. 2022, 61, e202116066; (b) Bhattacharjee, S.; Chongdar, S.; Modak, A.; Bhanja, P.; Jena, B. K.; Bhaumik, A. Synthesis of Isocyanate-Free Polyurethane Concocting Multiple Cyclic Carbonates Catalysed by a New Microporous Zinc Phosphonate via CO2 Fixation. Green Chem. 2022, 24, 8853–8862.
- 7(a) Yan, T.; Liu, H.; Zeng, Z. X.; Pan, W. G. Recent Progress of Catalysts for Synthesis of Cyclic Carbonates from CO2 and Epoxides. J. CO2 Util. 2023, 68, 102355; (b) Qiu, L.-Q.; Li, H.-R.; He, L.-N. Incorporating Catalytic Units into Nanomaterials: Rational Design of Multipurpose Catalysts for CO2 Valorization. Acc. Chem. Res. 2023, 56, 2225–2240.
- 8(a) Huang, R.; Rintjema, J.; González-Fabra, J.; Martín, E.; Escudero-Adán, E. C.; Bo, C.; Urakawa, A.; Kleij, A. W. Deciphering Key Intermediates in the Transformation of Carbon Dioxide into Heterocyclic Products. Nat. Catal. 2019, 2, 62–70; (b) Prasad, D.; Patil, K. N.; Chaudhari, N. K.; Kim, H.; Nagaraja, B. M.; Jadhav, A. H. Paving Way for Sustainable Earth-abundant Metal Based Catalysts for Chemical Fixation of CO2 into Epoxides for Cyclic Carbonate Formation. Catal. Rev. 2022, 64, 356–443.
- 9(a) Pal, T. K.; De, D.; Bharadwaj, P. K. Metal–Organic Frameworks for the Chemical Fixation of CO2 into Cyclic Carbonates. Coord. Chem. Rev. 2020, 408, 213173; (b) Zou, Y.-H.; Huang, Y.-B.; Si, D.-H.; Yin, Q.; Wu, Q.-J.; Weng, Z.; Cao, R. Porous Metal-Organic Framework Liquids for Enhanced CO2 Adsorption and Catalytic Conversion. Angew. Chem. Int. Ed. 2021, 60, 20915–20920.
- 10(a) Claver, C.; Yeamin, M. B.; Reguero, M.; Masdeu-Bultó, A. M. Recent Advances in the Use of Catalysts Based on Natural Products for the Conversion of CO2 into Cyclic Carbonates. Green Chem. 2020, 22, 7665–7706; (b) Zhang, A.; Chen, C.; Zuo, C.; Xu, X.; Cai, T.; Li, X.; Yuan, Y.; Yang, H.; Meng, G. Imidazolium-based Ionic Liquids Containing Multipoint Hydrogen Bond Donors as Bifunctional Organocatalysts for Efficient Cooperative Conversion of CO2 to Cyclic Carbonates. Green Chem. 2022, 24, 7194–7207; (c) Qiu, M.; Li, J.; Wu, H.; Huang, Y.; Guo, H.; Gao, D.; Shi, L.; Yi, Q. One-pot Non-covalent Heterogenization and Aromatization of Poly(ionic liquids) for Metal-/Cocatalyst- free and Atmospheric CO2 Conversion. Appl. Catal. B 2023, 322, 122125.
- 11(a) Liu, N.; Xie, Y.-F.; Wang, C.; Li, S.-J.; Wei, D.; Li, M.; Dai, B. Cooperative Multifunctional Organocatalysts for Ambient Conversion of Carbon Dioxide into Cyclic Carbonates. ACS Catal. 2018, 8, 9945–9957; (b) Zhang, Y.-Y.; Yang, G.-W.; Xie, R.; Yang, L.; Li, B.; Wu, G.-P. Scalable, Durable, and Recyclable Metal-Free Catalysts for Highly Efficient Conversion of CO2 to Cyclic Carbonates. Angew. Chem. Int. Ed. 2020, 59, 23291–23298; (c) Rostami, A.; Ebrahimi, A.; Al-Jassasi, M.; Mirzaei, S.; Al-Harrasi, A. 2-Picolinic Acid as a Naturally Occurring Hydrogen Bond Donor for the Preparation of Cyclic Carbonates from Terminal/Internal Epoxides and CO2. Green Chem. 2022, 24, 9069–9083.
- 12 Lidston, C. A. L.; Severson, S. M.; Abel, B. A.; Coates, G. W. Multifunctional Catalysts for Ring-Opening Copolymerizations. ACS Catal. 2022, 12, 11037–11070.
- 13 Ema, T.; Miyazaki, Y.; Shimonishi, J.; Maeda, C.; Hasegawa, J. Bifunctional Porphyrin Catalysts for the Synthesis of Cyclic Carbonates from Epoxides and CO2: Structural Optimization and Mechanistic Study. J. Am. Chem. Soc. 2014, 136, 15270–15279.
- 14(a) Maeda, C.; Taniguchi, T.; Ogawa, K.; Ema, T. Bifunctional Catalysts Based on M-phenylene-bridged Porphyrin Dimer and Trimer Platforms: Synthesis of Cyclic Carbonates from Carbon Dioxide and Epoxides. Angew. Chem. Int. Ed. 2015, 54, 134–138; (b) Jiang, X.; Gou, F.; Chen, F.; Jing, H. Cycloaddition of Epoxides and CO2 Catalyzed by Bisimidazole-functionalized Porphyrin Cobalt(III) Complexes. Green Chem. 2016, 18, 3567–3576; (c) Maeda, C.; Shimonishi, J.; Miyazaki, R.; Hasegawa, J.; Ema, T. Highly Active and Robust Metalloporphyrin Catalysts for the Synthesis of Cyclic Carbonates from a Broad Range of Epoxides and Carbon Dioxide. Chem. Eur. J. 2016, 22, 6556–6563; (d) Maeda, C.; Mitsuzane, M.; Ema, T. Chiral Bifunctional Metalloporphyrin Catalysts for Kinetic Resolution of Epoxides with Carbon Dioxide. Org. Lett. 2019, 21, 1853–1856; (e) Li, Z.; Su, Z.; Xu, W.; Shi, Q.; Yi, J.; Bai, C.; Wang, N.; Li, J. Cycloaddition Reactions of Epoxides and CO2 by the Novel Imidazolium-Functionalized Metalloporphyrins: Optimization and Analysis Using Response Surface Methodology. ChemCatChem 2020, 12, 4839–4844; (f) Dela Cruz, J. B.; Hung, C.-H. Ni and Zn N-confused Porphyrin Complexes as Recyclable Catalysts for High Efficiency Solvent-Free CO2 Fixation into Cyclic Carbonates. Catal. Sci. Technol. 2021, 11, 2144–2154; (g) Bai, D.; Wang, X.; Song, Y.; Li, B.; Zhang, L.; Yan, P.; Jing, H. Bifunctional Metalloporphyrins-Catalyzed Coupling Reaction of Epoxides and CO2 to Cyclic Carbonates. Chin. J. Catal. 2010, 31, 176–180; (h) Jiang, X.; Gou, F.; Fu, X.; Jing, H. Ionic Liquids-functionalized Porphyrins as Bifunctional Catalysts for Cycloaddition of Carbon Dioxide to Epoxides. J. CO2 Util. 2016, 16, 264–271.
- 15(a) Meléndez, J.; North, M.; Villuendas, P. One-Component Catalysts for Cyclic Carbonate Synthesis. Chem. Commun. 2009, 2577–2579; (b) Tian, D.; Liu, B.; Gan, Q.; Li, H.; Darensbourg, D. J. Formation of Cyclic Carbonates from Carbon Dioxide and Epoxides Coupling Reactions Efficiently Catalyzed by Robust, Recyclable One-Component Aluminum-Salen Complexes. ACS Catal. 2012, 2, 2029–2035; (c) Luo, R.; Zhou, X.; Chen, S.; Li, Y.; Zhou, L.; Ji, H. Highly Efficient Synthesis of Cyclic Carbonates from Epoxides Catalyzed by Salen Aluminum Complexes with Built-in “CO2 capture” Capability under Mild Conditions. Green Chem. 2014, 16, 1496–1506; (d) Martín, C.; Whiteoak, C. J.; Martin, E.; Martínez Belmonte, M.; Escudero-Adán, E. C.; Kleij, A. W. Easily Accessible Bifunctional Zn(salpyr) Catalysts for the Formation of Organic Carbonates. Catal. Sci. Technol. 2014, 4, 1615–1621; (e) Ren, W.-M.; Liu, Y.; Lu, X.-B. Bifunctional Aluminum Catalyst for CO2 Fixation: Regioselective Ring Opening of Three-membered Heterocyclic Compounds. J. Org. Chem. 2014, 79, 9771–9777; (f) Luo, R.; Yang, Z.; Zhang, W.; Zhou, X.; Ji, H. Recyclable Bifunctional Aluminum Salen Catalyst for CO2 Fixation: the Efficient Formation of Five-membered Heterocyclic Compounds. Sci. China Chem. 2017, 60, 979–989; (g) Zhang, W.; Luo, R.; Xu, Q.; Chen, Y.; Lin, X.; Zhou, X.; Ji, H. Transformation of Carbon Dioxide into Valuable Chemicals over Bifunctional Metallosalen Catalysts Bearing Quaternary Phosphonium Salts. Chin. J. Catal. 2017, 38, 736–744.
- 16(a) Rulev, Y. A.; Larionov, V. A.; Lokutova, A. V.; Moskalenko, M. A.; Lependina, O. G. L.; Maleev, V. I.; North, M.; Belokon, Y. N. Chiral Cobalt(III) Complexes as Bifunctional Brønsted Acid–Lewis Base Catalysts for the Preparation of Cyclic Organic Carbonates. ChemSusChem 2016, 9, 216–222; (b) Emelyanov, M. A.; Stoletova, N. V.; Lisov, A. A.; Medvedev, M. G.; Smol'yakov, A. F.; Maleev, V. I.; Larionov, V. A. An Octahedral Cobalt(III) Complex Based on Cheap 1,2-Phenylenediamine as a Bifunctional Metal-Templated Hydrogen Bond Donor Catalyst for Fixation of CO2 with Epoxides Under Ambient Conditions. Inorg. Chem. Front. 2021, 8, 3871–3884; (c) Virachotikul, A.; Laiwattanapaisarn, N.; Chainok, K.; Phomphrai, K. Bifunctional Zinc and Magnesium Schiff-Base Complexes Containing Quaternary Ammonium Side-Arms for Epoxide/CO2 Coupling Reactions. Dalton Trans. 2021, 50, 12399–12403.
- 17(a) De La Cruz-Martínez, F.; Martínez, J.; Gaona, M. A.; Fernández-Baeza, J.; Sánchez-Barba, L. F.; Rodríguez, A. M.; Castro-Osma, J. A.; Otero, A.; Lara-Sánchez, A. Bifunctional Aluminum Catalysts for the Chemical Fixation of Carbon Dioxide into Cyclic Carbonates. ACS Sustainable Chem. Eng. 2018, 6, 5322–5332; (b) Della Monica, F.; Buonerba, A.; Paradiso, V.; Milione, S.; Grassi, A.; Capacchione, C. [OSSO]-Type Fe(III) Metallate as Single-Component Catalyst for the CO2 Cycloaddition to Epoxides. Adv. Synth. Catal. 2019, 361, 283–288; (c) Martínez, J.; De La Cruz-Martínez, F.; Gaona, M. A.; Pinilla-Peñalver, E.; Fernández-Baeza, J.; Rodríguez, A. M.; Castro-Osma, J. A.; Otero, A.; Lara-Sánchez, A. Influence of the Counterion on the Synthesis of Cyclic Carbonates Catalyzed by Bifunctional Aluminum Complexes. Inorg. Chem. 2019, 58, 3396–3408; (d) Naveen, K.; Ji, H.; Kim, T. S.; Kim, D.; Cho, D.-H. C3-symmetric Zinc Complexes as Sustainable Catalysts for Transforming Carbon Dioxide into Mono- and Multi-Cyclic Carbonates. Appl. Catal. B 2021, 280, 119395; (e) Wei, F.; Tang, J.; Zuhra, Z.; Wang, S.; Wang, X.; Wang, X.; Xie, G. [M(Me6Tren)X]X Complex as Efficacious Bifunctional Catalyst for CO2 Cycloaddition: the Synergism of the Metal and Halogen Ions. J. CO2 Util. 2022, 61, 102048; (f) Wang, B.; Wang, L.; Lin, J.; Xia, C.; Sun, W. Multifunctional Zn-N4 Catalysts for the Coupling of CO2 with Epoxides into Cyclic Carbonates. ACS Catal. 2023, 13, 10386–10393; (g) Alonso de la Peña, M.; Merzoud, L.; Bussé-Côté, D.; Tuel, A.; Morell, C.; Chermette, H.; Christ, L. Zwitterionic Bifunctional Stable Zinc Complexes as Single Catalysts for the Valorization of CO2 into Cyclic Carbonates. Mol. Catal. 2024, 553, 113746; (h) Martínez, J.; Castro-Osma, J. A.; Alonso-Moreno, C.; Rodríguez-Diéguez, A.; North, M.; Otero, A.; Lara-Sánchez, A. One-Component Aluminum(heteroscorpionate) Catalysts for the Formation of Cyclic Carbonates from Epoxides and Carbon Dioxide. ChemSusChem 2017, 10, 1175–1185.
- 18 Arnold, P. L.; Kerr, R. W. F.; Weetman, C.; Docherty, S. R.; Rieb, J.; Cruickshank, F. L.; Wang, K.; Jandl, C.; McMullon, M. W.; Pöthig, A.; Kühn, F. E.; Smith, A. D. Selective and Catalytic Carbon Dioxide and Heteroallene Activation Mediated by Cerium N-heterocyclic Carbene Complexes. Chem. Sci. 2018, 9, 8035–8045.
- 19(a) Bayer, U.; Werner, D.; Maichle-Mössmer, C.; Anwander, R. Effective and Reversible Carbon Dioxide Insertion into Cerium Pyrazolates. Angew. Chem. Int. Ed. 2020, 59, 5830–5836; (b) Sun, X.; Yuan, K.; Zhang, Y. Advances and Prospects of Rare Earth Metal-Organic Frameworks in Catalytic Applications. J. Rare Earths 2020, 38, 801–818.
- 20(a) Xin, X.; Shan, H.; Tian, T.; Wang, Y.; Yuan, D.; You, H.; Yao, Y. Conversion of CO2 into Cyclic Carbonates under Ambient Conditions Catalyzed by Rare-Earth Metal Complexes Bearing Poly(phenolato) Ligand. ACS Sustainable Chem. Eng. 2020, 8, 13185–13194; (b) Qing, Y.; Liu, T.; Zhao, B.; Bao, X.; Yuan, D.; Yao, Y. Cycloaddition of Di-Substituted Epoxides and CO2 under Ambient Conditions Catalysed by Rare-Earth Poly(phenolate) Complexes. Inorg. Chem. Front. 2022, 9, 2969–2979; (c) Yin, K.; Hua, L.; Qu, L.; Yao, Q.; Wang, Y.; Yuan, D.; You, H.; Yao, Y. Heterobimetallic Rare Earth Metal–Zinc Catalysts for Reactions of Epoxides and CO2 under Ambient Conditions. Dalton Trans. 2021, 50, 1453–1464; (d) Yao, Q.; Wang, Y.; Zhao, B.; Zhu, X.; Luo, Y.; Yuan, D.; Yao, Y. Syntheses of Heterometallic Neodymium-Zinc Complexes and Their Performance in the Copolymerization of CO2 and Cyclohexene Oxide. Inorg. Chem. 2022, 61, 10373–10382; (e) Yao, Q.; Chen, Y.; Wang, Y.; Yuan, D.; You, H.; Yao, Y. Alternating Copolymerization of CO2 and Cyclohexene Oxide Initiated by Rare-Earth Metal Complexes Stabilized by O-phenylenediamine-bridged Tris(phenolate) Ligand. J. Rare Earths 2022, 40, 1471–1479; (f) Feng, C.; Mu, D.; Zhu, X.; Wang, Y.; Yuan, D.; Yao, Y. Synthesis of Benzoxazine Functionalized Amine-Bridged Bis(phenolato) Rare Earth Complexes and Their Application in Ring-Opening Polymerization of rac-Lactide. Chin. J. Chem. 2022, 40, 2516–2524; (g) Song, Y.; Yin, K.; Chen, Y.; Zhao, B.; Zhang, Y.; Zhu, X.; Yuan, D.; Yao, Y. Synthesis of Heterometallic Rare Earth(III)–Cobalt(II) Complexes and Their Application in Alternating Copolymerization of Cyclohexene Oxide and Carbon Dioxide. Chin. J. Chem. 2023, 41, 805–813.
- 21(a) Dong, Y.; Chang, K.; Xu, X. Reactions of Rare-Earth Metal Based Lewis Pairs with Azides. Chin. J. Chem. 2020, 38, 559–564; (b) Zhou, Y.; Jiang, S.; Xu, X. Isospecific Polymerization of Methyl Methacrylate by Intramolecular Rare-Earth Metal Based Lewis Pairs. Chin. J. Chem. 2021, 39, 149–156.
- 22 Bokouende, S. S.; Kulasekara, D. N.; Worku, S. A.; Ward, C. L.; Kajjam, A. B.; Lutter, J. C.; Allen, M. J. Expanding the Coordination of f-Block Metals with Tris[2-(2-methoxyethoxy)ethyl]amine: From Molecular Complexes to Cage-Like Structures. Inorg. Chem. 2023, DOI: https://doi.org/10.1021/acs.inorgchem.1023c02752.
- 23 Deacon, G. B.; Feng, T. C.; Junk, P. C.; Meyer, G.; Scott, N. M.; Skelton, B. W.; White, A. H. Structural Variety in Solvated Lanthanoid(III) Halide Complexes. Aust. J. Chem. 2000, 53, 853–865.
- 24 Roitershtein, D.; Domingos, Â.; Pereira, L. C. J.; Ascenso, J. R.; Marques, N. Coordination of 2,2‘-Bipyridyl and 1,10-Phenanthroline to Yttrium and Lanthanum Complexes Based on a Scorpionate Ligand. Inorg. Chem. 2003, 42, 7666–7673.
- 25 Qin, J.; Wang, P.; Li, Q.; Zhang, Y.; Yuan, D.; Yao, Y. Catalytic Production of Cyclic Carbonates Mediated by Lanthanide Phenolates under Mild Conditions. Chem. Commun. 2014, 50, 10952–10955.
- 26 Whiteoak, C. J.; Gjoka, B.; Martin, E.; Belmonte, M. M.; Escudero-Adán, E. C.; Zonta, C.; Licini, G.; Kleij, A. W. Reactivity Control in Iron(III) Amino Triphenolate Complexes: Comparison of Monomeric and Dimeric Complexes. Inorg. Chem. 2012, 51, 10639–10649.
- 27(a) Zhao, Z.; Qin, J.; Zhang, C.; Wang, Y.; Yuan, D.; Yao, Y. Recyclable Single-component Rare-Earth Metal Catalysts for Cycloaddition of CO2 and Epoxides at Atmospheric Pressure. Inorg. Chem. 2017, 56, 4568–4575; (b) Yao, Q.; Shi, Y.; Wang, Y.; Zhu, X.; Yuan, D.; Yao, Y. Bifunctional Rare-Earth Metal Catalysts for Conversion of CO2 and Epoxides into Cyclic Carbonates. Asian J. Org. Chem. 2022, 11, e202200106.
- 28 Martínez, J.; Fernández-Baeza, J.; Sánchez-Barba, L. F.; Castro-Osma, J. A.; Lara-Sánchez, A.; Otero, A. An Efficient and Versatile Lanthanum Heteroscorpionate Catalyst for Carbon Dioxide Fixation into Cyclic Carbonates. ChemSusChem 2017, 10, 2886–2890.
- 29 Han, Q.; Wang, L.; Shi, Z.; Xu, C.; Dong, Z.; Mou, Z.; Liu, W. Self-Assembly of Luminescent Lanthanide Mesocates as Efficient Catalysts for Transforming Carbon Dioxide into Cyclic Carbonates. Chem. Asian J. 2017, 12, 1364–1373.
- 30 Cheng, J.; Lu, C.; Zhao, B. Cycloaddition of Carbon Dioxide and Epoxides Catalyzed by Rare Earth Metal Complexes Bearing a Trost Ligand. New J. Chem. 2021, 45, 13096–13103.
- 31(a) Xu, B.; Wang, P.; Lv, M.; Yuan, D.; Yao, Y. Transformation of Carbon Dioxide into Oxazolidinones and Cyclic Carbonates Catalyzed by Rare-earth-metal Phenolates. ChemCatChem 2016, 8, 2466–2471; (b) Hua, L.; Li, B.; Han, C.; Gao, P.; Wang, Y.; Yuan, D.; Yao, Y. Synthesis of Homo- and Heteronuclear Rare-earth Metal Complexes Stabilized by Ethanolamine-bridged Bis(phenolato) Ligands and Their Application in Catalyzing Reactions of CO2 and Epoxides. Inorg. Chem. 2019, 58, 8775–8786.
- 32(a) Peng, J.; Yang, H.-J.; Wang, S.; Ban, B.; Wei, Z.; Lei, B.; Guo, C.-Y. Efficient Solvent-Free Fixation of CO2 Catalyzed by New Recyclable Bifunctional Metal Complexes. J. CO2 Util. 2018, 24, 1–9; (b) Hu, Y.; Wei, Z.; Frey, A.; Kubis, C.; Ren, C.-Y.; Spannenberg, A.; Jiao, H.; Werner, T. Catalytic, Kinetic, and Mechanistic Insights into the Fixation of CO2 with Epoxides Catalyzed by Phenol-Functionalized Phosphonium Salts. ChemSusChem 2021, 14, 363–372.
- 33 Darensbourg, D. J.; Fitch, S. B. An Exploration of the Coupling Reactions of Epoxides and Carbon Dioxide Catalyzed by Tetramethyltetraazaannulene Chromium(III) Derivatives: Formation of Copolymers Versus Cyclic Carbonates. Inorg. Chem. 2008, 47, 11868–11878.




