Immunological Exploration of Helicobacter pylori Serotype O2 O-Antigen by Using a Synthetic Glycan Library
Ming Zhao
Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology & School of Life Sciences and Health Engineering, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
These authors contributed equally.
Search for more papers by this authorGuangzong Tian
Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology & School of Life Sciences and Health Engineering, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
These authors contributed equally.
Search for more papers by this authorChunjun Qin
Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology & School of Life Sciences and Health Engineering, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorXiaopeng Zou
Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology & School of Life Sciences and Health Engineering, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorPeter H. Seeberger
Biomolecular Systems Department, Max Planck Institute of Colloids and Interfaces, Am Mühlenberg 1, Potsdam, 14476 Germany
Search for more papers by this authorCorresponding Author
Jing Hu
Wuxi School of Medicine, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jian Yin
Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology & School of Life Sciences and Health Engineering, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMing Zhao
Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology & School of Life Sciences and Health Engineering, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
These authors contributed equally.
Search for more papers by this authorGuangzong Tian
Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology & School of Life Sciences and Health Engineering, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
These authors contributed equally.
Search for more papers by this authorChunjun Qin
Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology & School of Life Sciences and Health Engineering, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorXiaopeng Zou
Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology & School of Life Sciences and Health Engineering, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
Search for more papers by this authorPeter H. Seeberger
Biomolecular Systems Department, Max Planck Institute of Colloids and Interfaces, Am Mühlenberg 1, Potsdam, 14476 Germany
Search for more papers by this authorCorresponding Author
Jing Hu
Wuxi School of Medicine, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jian Yin
Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology & School of Life Sciences and Health Engineering, Jiangnan University, Lihu Avenue 1800, Wuxi, Jiangsu, 214122 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
Helicobacter pylori (H. pylori) infection is a threat to human health. The lipopolysaccharide (LPS) O-antigen holds promise for developing vaccines. It is meaningful to explore the immunological activity of oligosaccharides with different lengths and frameshifts for antigen development. Herein, a glycan library of H. pylori O2 O-antigen containing eight fragments is constructed. After screening with anti-H. pylori O2 LPS sera and patients’ sera by glycan microarray, the disaccharide HPO2G-2b and trisaccharide HPO2G-3a show strong antigenicity and then are separately conjugated with carrier protein CRM197. Both glycoconjugates elicit a robust immunoglobulin G (IgG) immune response in rabbits. The anti-HPO2G-3a IgG antibodies possess a much stronger binding affinity with the LPS and bacteria of H. pylori O2 than the anti-HPO2G-2b IgG antibodies. There is no cross-reaction between both sera IgG antibodies with LPS and bacteria of H. pylori O1, O6, and E. coli. The results demonstrate the trisaccharide HPO2G-3a is a promising candidate for H. pylori vaccine development.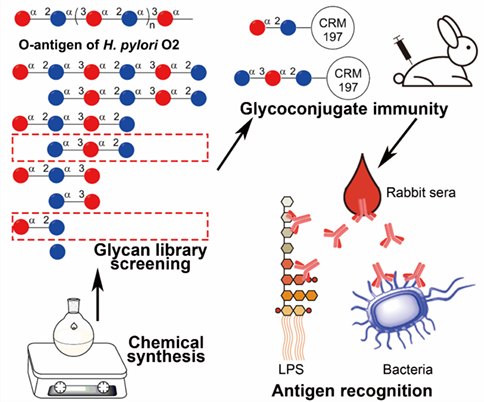
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300510-sup-0001-supinfo.pdfPDF document, 5.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Zhong, Y. X.; Chen, J.; Liu, Y.; Zhang, Y. B.; Tang, C. F.; Wang, X. W.; Wang, P.; Chen, W. X.; Wei, B.; Liu, M. Y. Oral immunization of BALB/c mice with recombinant Helicobacter pylori antigens and double mutant heat-labile toxin (dmLT) induces prophylactic protective immunity against H. pylori infection. Microb. Pathog. 2020, 145, 104229.
- 2 Strugatsky, D.; McNulty, R.; Munson, K.; Chen, C. K.; Soltis, S. M.; Sachs, G.; Luecke, H. Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori. Nature 2013, 493, 255–258.
- 3 Franceschi, F.; Zuccala, G.; Roccarina, D.; Gasbarrini, A. Clinical effects of Helicobacter pylori outside the stomach. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 234–242.
- 4 Maldonado, R. F.; Sa-Correia, I.; Valvano, M. A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. Fems Microbiol. Rev. 2016, 40, 480–493.
- 5 Zou, X. P.; Hu, J.; Zhao, M.; Qin, C. J.; Zhu, Y. T.; Tian, G. Z.; Cai, J. T.; Seeberger, P. H.; Yin, J. Chemical Synthesis of the Highly Sterically Hindered Core Undecasaccharide of Helicobacter pylori Lipopolysaccharide for Antigenicity Evaluation with Human Serum. J. Am. Chem. Soc. 2022, 144, 14535–14547.
- 6 Flores-Luna, L.; Bravo, M. M.; Kasamatsu, E.; Lazcano Ponce, E. C.; Martinez, T.; Torres, J.; Camorlinga-Ponce, M.; Kato, I. Risk factors for gastric precancerous and cancers lesions in Latin American counties with difference gastric cancer risk. Cancer Epidemiol. 2020, 64, 101630.
- 7(a) Safavi, M.; Sabourian, R.; Foroumadi, A. Treatment of Helicobacter pylori infection: Current and future insights. World J. Clin. Cases 2016, 4, 5–19; (b) Liang, B.; Yuan, Y.; Peng, X. J.; Liu, X. L.; Hu, X. K.; Xing, D. M. Current and future perspectives for Helicobacter pylori treatment and management: From antibiotics to probiotics. Front. Cell. Infect. Microbiol. 2022, 12, 1042070.
- 8 Hu, Y.; Zhu, Y.; Lu, N. H. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 168.
- 9 Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629.
- 10(a) Wang, J. C.; Zhang, Y. Y.; Zhu, Y. R.; Liu, J. R.; Chen, Y.; Cao, X.; Yang, Y. Total Synthesis and Immunological Evaluation of the Tri-D-glycero-D-manno-heptose Antigen of the Lipopolysaccharide as a Vaccine Candidate against Helicobacter pylori. Org. Lett. 2020, 22, 8780–8785; (b) Altman, E.; Chandan, V.; Harrison, B. A.; Vinogradov, E. Structural and immunological characterization of a glycoconjugate based on the delipidated lipopolysaccharide from a nontypeable Helicobacter pylori strain PJ1 containing an extended D-glycero-D- manno-heptan. Carbohydr. Res. 2018, 456, 19–23; (c) Altman, E.; Chandan, V.; Harrison, B. A.; Veloso-Pita, R.; Li, J.; KuoLee, R.; Chen, W.; Verez-Bencomo, V. Regional Helicobacter pylori Study, G. Design and immunological properties of Helicobacter pylori glycoconjugates based on a truncated lipopolysaccharide lacking Lewis antigen and comprising an alpha-1,6-glucan chain. Vaccine 2012, 30, 7332–7341; (d) Monteiro, M. A.; Britton, S.; Applebee, L. A.; Baqar, S. Synthesis and immunogenicity of a Helicobacter pylori lipopolysaccharide- based conjugate. Vaccine 2011, 29, 3098–3102; (e) Dos Santos Viana, I.; Cordeiro Santos, M. L.; Santos Marques, H.; Lima de Souza Goncalves, V.; Bittencourt de Brito, B.; Franca da Silva, F. A.; Oliveira, E. S. N.; Dantas Pinheiro, F.; Fernandes Teixeira, A.; Tanajura Costa, D.; Oliveira Souza, B.; Lima Souza, C.; Vasconcelos Oliveira, M.; Freire de Melo, F. Vaccine development against Helicobacter pylori: from ideal antigens to the current landscape. Expert Rev. Vaccines 2021, 20, 989–999.
- 11 Mills, S. D.; Kurjanczyk, L. A.; Penner, J. L. Antigenicity of Helicobacter pylori lipopolysaccharides. J. Clin. Microbiol. 1992, 30, 3175–3180.
- 12 Simoons-Smit, I. M.; Appelmelk, B. J.; Verboom, T.; Negrini, R.; Penner, J. L.; Aspinall, G. O.; Moran, A. P.; Fei, S. F.; Shi, B. S.; Rudnica, W.; Savio, A.; de Graaff, J. Typing of Helicobacter pylori with monoclonal antibodies against Lewis antigens in Lipopolysaccharide. J. Clin. Microbiol. 1996, 34, 2196–2200.
- 13
Ferreira, J. A.; Silva, L.; Monteiro, M. A.; Coimbra, M. A. Helicobacter pylori cell-surface glycans structural features: role in gastric colonization, pathogenesis, and carbohydrate-based vaccines. In Carbohydrate Chemistry, The Royal Society of Chemistry, London, 2011, pp. 160–193.
10.1039/9781849732765-00160 Google Scholar
- 14 Esmaeilli, D.; Mobarez, A. M.; Salmanian, A. H.; Hosseini, A. Z. Bioactivity and immunological evaluation of LPS from different serotypes of Helicobacter pylori. Iran. J. Microbiol. 2013, 5, 142–146.
- 15 Britton, S.; Papp-Szabo, E.; Simala-Grant, J.; Morrison, L.; Taylor, D. E.; Monteiro, M. A. A novel Helicobacter pylori cell-surface polysaccharide. Carbohydr. Res. 2005, 340, 1605–1611.
- 16(a) Anish, C.; Schumann, B.; Pereira, C. L.; Seeberger, P. H. Chemical biology approaches to designing defined carbohydrate vaccines. Chem. Biol. 2014, 21, 38–50; (b) Mettu, R.; Lih, Y. H.; Vulupala, H. R.; Chen, C. Y.; Hsu, M. H.; Lo, H. J.; Liao, K. S.; Cheng, Y. Y.; Chiu, C. H.; Wu, C. Y. Synthetic Library of Oligosaccharides Derived from the Capsular Polysaccharide of Streptococcus pneumoniae Serotypes 6A and 6B and Their Immunological Studies. ACS Infect. Dis. 2022, 8, 626–634; (c) Sianturi, J.; Priegue, P.; Hu, J.; Yin, J.; Seeberger, P. H. Semi-Synthetic Glycoconjugate Vaccine Lead Against Acinetobacter baumannii 17978. Angew. Chem. Int. Ed. 2022, 61, e202209556; (d) Cohen, D.; Atsmon, J.; Artaud, C.; Meron-Sudai, S.; Gougeon, M. L.; Bialik, A.; Goren, S.; Asato, V.; Ariel-Cohen, O.; Reizis, A.; Dorman, A.; Hoitink, C. W. G.; Westdijk, J.; Ashkenazi, S.; Sansonetti, P.; Mulard, L. A.; Phalipon, A. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: a phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis. 2021, 21, 546–558.
- 17 Verez-Bencomo, V.; Fernandez-Santana, V.; Hardy, E.; Toledo, M. E.; Rodriguez, M. C.; Heynngnezz, L.; Rodriguez, A.; Baly, A.; Herrera, L.; Izquierdo, M.; Villar, A.; Valdes, Y.; Cosme, K.; Deler, M. L.; Montane, M.; Garcia, E.; Ramos, A.; Aguilar, A.; Medina, E.; Torano, G.; Sosa, I.; Hernandez, I.; Martinez, R.; Muzachio, A.; Carmenates, A.; Costa, L.; Cardoso, F.; Campa, C.; Diaz, M.; Roy, R. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science 2004, 305, 522–525.
- 18 Tian, G. Z.; Qin, C. J.; Liu, Z. H.; Shen, D. C.; Zou, X. P.; Fu, J. J.; Hu, J.; Seeberger, P. H.; Yin, J. Total synthesis of the Helicobacter pylori serotype O2 O-antigen alpha-(1 -> 2)- and alpha-(1 -> 3)-linked oligoglucosides. Chem. Commun. 2020, 56, 344–347.
- 19 Cai, J. T.; Hu, J.; Qin, C. J.; Li, L. X.; Shen, D. C.; Tian, G. Z.; Zou, X. P.; Seeberger, P. H.; Yin, J. Chemical Synthesis Elucidates the Key Antigenic Epitope of the Autism-Related Bacterium Clostridium bolteae Capsular Octadecasaccharide. Angew. Chem. Int. Ed. 2020, 59, 20529–20537.
- 20 Schumann, B.; Hahm, H. S.; Parameswarappa, S. G.; Reppe, K.; Wahlbrink, A.; Govindan, S.; Kaplonek, P.; Pirofski, L. A.; Witzenrath, M.; Anish, C.; Pereira, C. L.; Seeberger, P. H. A semisynthetic Streptococcus pneumoniae serotype 8 glycoconjugate vaccine. Sci. Transl. Med. 2017, 9, eaaf5347.
- 21 Johnson, M. A.; Cartmell, J.; Weisser, N. E.; Woods, R. J.; Bundle, D. R. Molecular Recognition of Candida albicans (1 -> 2)-beta-Mannan Oligosaccharides by a Protective Monoclonal Antibody Reveals the Immunodominance of Internal Saccharide Residues. J. Biol. Chem. 2012, 287, 18078–18090.
- 22 Komarova, B. S.; Tsvetkov, Y. E.; Nifantiev, N. E. Design of α-selective glycopyranosyl donors relying on remote anchimeric assistance. Chem. Rec. 2016, 16, 488–506.
- 23 Qin, C. J.; Schumann, B.; Zou, X. P.; Pereira, C. L.; Tian, G. Z.; Hu, J.; Seeberger, P. H.; Yin, J. Total Synthesis of a Densely Functionalized Plesiomonas shigelloides Serotype 51 Aminoglycoside Trisaccharide Antigen. J. Am. Chem. Soc. 2018, 140, 3120–3127.
- 24 Aspinall, G. O.; Monteiro, M. A.; Pang, H.; Walsh, E. J.; Moran, A. P. Lipopolysaccharide of the Helicobacter pylori type strain NCTC 11637 (ATCC 43504): Structure of the O antigen chain and core oligosaccharide regions. Biochemistry 1996, 35, 2489–2497.
- 25 Chen, R. C.; Shang, H. Q.; Niu, X. Y.; Huang, J.; Miao, Y. Q.; Sha, Z.; Qin, L. T.; Huang, H.; Peng, D.; Zhu, R. L. Establishment and evaluation of an indirect ELISA for detection of antibodies to goat Klebsiella pneumonia. BMC Vet. Res. 2021, 17, 107.
- 26(a) Martin, C. E.; Broecker, F.; Oberli, M. A.; Komor, J.; Mattner, J.; Anish, C.; Seeberger, P. H. Immunological Evaluation of a Synthetic Clostridium difficile Oligosaccharide Conjugate Vaccine Candidate and Identification of a Minimal Epitope. J. Am. Chem. Soc. 2013, 135, 9713–9722; (b) Tian, G. Z.; Hu, J.; Qin, C. J.; Li, L. X.; Zou, X. P.; Cai, J. T.; Seeberger, P. H.; Yin, J. Chemical Synthesis and Immunological Evaluation of Helicobacter pylori Serotype O6 Tridecasaccharide O-Antigen Containing a dd-Heptoglycan. Angew. Chem. Int. Ed. 2020, 59, 13362–13370.
- 27 Ding, S. Z.; Du, Y. Q.; Lu, H.; Wang, W. H.; Cheng, H.; Chen, S. Y.; Chen, M. H.; Chen, W. C.; Chen, Y.; Fang, J. Y.; Gao, H. J.; Guo, M. Z.; Han, Y.; Hou, X. H.; Hu, F. L.; Jiang, B.; Jiang, H. X.; Lan, C. H.; Li, J. N.; Li, Y.; Li, Y. Q.; Liu, J.; Li, Y. M.; Lyu, B.; Lu, Y. Y.; Miao, Y. L.; Nie, Y. Z.; Qian, J. M.; Sheng, J. Q.; Tang, C. W.; Wang, F.; Wang, H. H.; Wang, J. B.; Wang, J. T.; Wang, J. P.; Wang, X. H.; Wu, K. C.; Xia, X. Z.; Xie, W. F.; Xie, Y.; Xu, J. M.; Yang, C. Q.; Yang, G. B.; Yuan, Y.; Zeng, Z. R.; Zhang, B. Y.; Zhang, G. Y.; Zhang, G. X.; Zhang, J. Z.; Zhang, Z. Y.; Zheng, P. Y.; Zhu, Y.; Zuo, X. L.; Zhou, L. Y.; Lyu, N. H.; Yang, Y. S.; Li, Z. S.; National Clinical Research Center for Digestive Diseases (Shanghai); Gastrointestinal Early Cancer Prevention & Treatment Alliance of China (GECA); Helicobacter pylori Study Group of Chinese Society of Gastroenterology; Chinese Alliance for Helicobacter pylori Study. Chinese Consensus Report on Family-Based Helicobacter pylori Infection Control and Management (2021 Edition). Gut 2022, 71, 238–253.
- 28 Zhou, X. Z.; Lyu, N. H.; Zhu, H. Y.; Cai, Q. C.; Kong, X. Y.; Xie, P.; Zhou, L. Y.; Ding, S. Z.; Li, Z. S.; Du, Y. Q.; National Clinical Research Center for Digestive Diseases (Shanghai); Gastrointestinal Early Cancer Prevention & Treatment Alliance of China (GECA); Helicobacter pylori Study Group of Chinese Society of Gastroenterology; Chinese Alliance for Helicobacter pylori Study. Large-scale, national, family-based epidemiological study on Helicobacter pylori infection in China: the time to change practice for related disease prevention. Gut 2023, 72, 855–869.
- 29 van Diepen, A.; van der Plas, A. J.; Kozak, R. P.; Royle, L.; Dunne, D. W.; Hokke, C. H. Development of a Schistosoma mansoni shotgun O-glycan microarray and application to the discovery of new antigenic schistosome glycan motifs. Int. J. Parasitol. 2015, 45, 465–475.
- 30 Zirk, K.; Poetzschke, H. On the suitability of refractometry for the analysis of glucose in blood-derived fluids. Med. Eng. Phys. 2004, 26, 473–481.
- 31 Avci, F. Y.; Li, X. M.; Tsuji, M.; Kasper, D. L. Carbohydrates and T cells: A sweet twosome. Semin. Immunol. 2013, 25, 146–151.
- 32 Kobayashi, M.; Farrar, J. L.; Gierke, R.; Leidner, A. J.; Campos-Outcalt, D.; Morgan, R. L.; Long, S. S.; Poehling, K. A.; Cohen, A. L. Use of 15-Valent Pneumococcal Conjugate Vaccine Among US Children: Updated Recommendations of the Advisory Committee on Immunization Practices - United States, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1174–1181.
- 33 Kaplonek, P.; Khan, N.; Reppe, K.; Schumann, B.; Emmadi, M.; Lisboa, M. P.; Xu, F. F.; Calow, A. D. J.; Parameswarappa, S. G.; Witzenrath, M.; Pereira, C. L.; Seeberger, P. H. Improving vaccines against Streptococcus pneumoniae using synthetic glycans. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 13353–13358.
- 34 Boes, M. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 2000, 37, 1141–1149.
- 35 Qin, C. J.; Li, L. X.; Tian, G. Z.; Ding, M. R.; Zhu, S. Y.; Song, W. Q.; Hu, J.; Seeberger, P. H.; Yin, J. Chemical Synthesis and Antigenicity Evaluation of Shigella dysenteriae Serotype 10 O-Antigen Tetrasaccharide Containing a Ketal. J. Am. Chem. Soc. 2022, 144, 21068–21079.
- 36(a) Simpson, B. W.; Trent, M. S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019, 17, 403–416; (b) Zhao, M.; Qin, C. J.; Li, L. X.; Xie, H. T.; Ma, B. N.; Zhou, Z. R.; Yin, J.; Hu, J. Conjugation of Synthetic Trisaccharide of Staphylococcus aureus Type 8 Capsular Polysaccharide Elicits Antibodies Recognizing Intact Bacterium. Front. Chem. 2020, 8, 258.
- 37 Gildersleeve, J. C.; Wright, W. S. Diverse molecular recognition properties of blood group A binding monoclonal antibodies. Glycobiology 2016, 26, 443–448.




