Isocyanide-Based One-Pot Cascade Synthesis of 3-Acyl Isoindolinones†
Jia Liu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorYi-Ming Zhu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Xiao-Ping Xu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Shun-Jun Ji
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu, 215123 China
Suzhou Baolidi Functional Materials Research Institute, Suzhou, Jiangsu, 215144 China
E-mail: [email protected]; [email protected]Search for more papers by this authorJia Liu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorYi-Ming Zhu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Xiao-Ping Xu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu, 215123 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Shun-Jun Ji
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu, 215123 China
Suzhou Baolidi Functional Materials Research Institute, Suzhou, Jiangsu, 215144 China
E-mail: [email protected]; [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
A series of 3-acyl-substituted isoindolinone derivatives were synthesized in a one-pot manner via the reaction of o-bromobenzaldehydes, isocyanides, and carboxylic acids in the presence of palladium catalyst and base. The reaction employing easily available starting materials features simple operation and high efficiency. The mechanistic study showed that the reaction might undergo 1) Pd-catalyzed [3+2] cyclization of o-bromobenzaldehyde with isocyanide and the re-insertion of another molecule of isocyanide, 2) addition of carboxylic acid to in situ formed ketenimine followed by a rearrangement relay to give 3,3-diacyl-substituted isoindolinone derivative. Further transformations of the obtained products through decarbonylation could also be realized.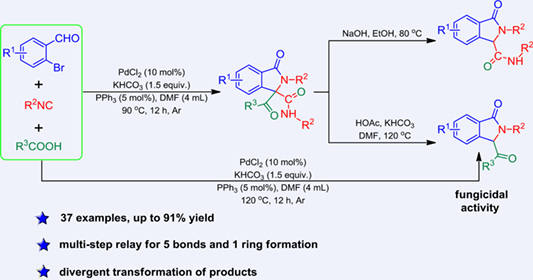
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300488-sup-0001-supinfo.pdfPDF document, 8.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Xu, Y.; Liu, X.-Y.; Wang, Z.-H.; Tang, L.-F. Synthesis of 3-acyl, methylene and epoxy substituted isoindolinone derivatives via the ortho-lithiation/cyclization procedures of aromatic imines with carbon monoxide. Tetrahedron 2017, 73, 7245–7253; (b) Johan, K.; Ingemar, J.; Annika, Å.; Roine, I. O.; Ulrik, G.; Annika, B.; Ola, F.; Öjvind, D.; Hans, E.; Anders, D.; Boel, L.; Yuan, Z.-Q.; Johan, S.; Johan, C.; Jonna, G.; Tommy, I.; Ågot, H.; Emma, L.; Jesper, M. Isoindolinone compounds active as Kv1.5 blockers identified using a multicomponent reaction approach. Bioorg. Med. Chem. Lett. 2016, 26, 2023–2029; (c) Macsari, I.; Besidski, Y.; Csjernyik, G.; Nilsson, L. I.; Sandberg, L.; Yngve, U.; Åhlin, K.; Bueters, T.; Eriksson, A. B.; Lund, P. E.; Venyike, E.; Oerther, S.; Blakeman, K. H.; Luo, L.; Arvidsson, P. I. 3-Oxoisoindoline-1-carboxamides: Potent, State-Dependent Blockers of Voltage-Gated Sodium Channel NaV1.7 with Efficacy in Rat Pain Models. J. Med. Chem. 2012, 55, 6866−6880.
- 2(a) Sultane, P. R.; Ahumada, G.; Daniel, J.-M.; Bielawski, C. W. Cyclic (Aryl)(Amido)Carbenes: NHCs with Triplet-like Reactivity. Angew. Chem. Int. Ed. 2019, 58, 16320–16325; (b) Couture, A.; Deniau, E.; Ionescu, D.; Grandclaudon, P. LDA-induced Metalation of Isoindolinones. An Efficient Route to 3-Substituted Isoindoline Derivatives. Tetrahedron Lett. 1998, 39, 2319–2320.
- 3(a) Wacker, D. A.; Varnes, J. G.; Malmstrom, S. E.; Cao, X.-Y.; Hung, C. P.; Ung, T.; Wu, G.; Zhang, G.; Zuvich, E.; Thomas, M. A.; Keim, W. J.; Cullen, M. J.; Rohrbach, K. W.; Qu, Q.-L.; Narayanan, R.; Rossi, K.; Janovitz, E.; McKeeman, L. L.; Malley, M. F.; Devenny, J.; Pelleymounter, M. A.; Miller, K. J.; Robl, J. A. Discovery of (R)-9-Ethyl-1,3,4,10b-tetrahydro-7-trifluoromethylpyrazino[2,1-a]isoindol-6(2H)-one, a Selective, Orally Active Agonist of the 5-HT2C Receptor. J. Med. Chem. 2007, 50, 1365–1379; (b) Klíma, J.; Polášek, M.; Ludvík, J.; Urban, J. Reaction of Phthalaldehyde with Aminoethanol under Different Conditions: Products and Mechanisms of Their Formation. J. Hetercycl. Chem. 2012, 49, 1202–1209.
- 4 Chen, W.-L.; Jin, L.-N.; Zhu, Y.-H.; Cao, X.-J.; Zheng, L.-B.; Mo, W.-M. Palladium-Catalyzed Intramolecular Cyclization of 2-Iodobenzamides: An Efficient Synthesis of 3-Acyl Isoindolin-1-ones and 3-Hydroxy-3- acyliso-indolin-1-ones. Synlett 2013, 24, 1856–1860.
- 5(a) Schmitt, G.; An, N. D.; Poupeln, J. P.; Vebrel, J.; Laude, B. A New and Mild Synthesis of Substituted Salicylic Acids. Synthesis 1984, 9, 758–760;
10.1055/s-1984-30960 Google Scholar(b) Kundu, A.; Pathak, S.; Debnath, K.; Pramanik, A.; Facile synthesis of 3H,3’H-spiro[benzofuran-2,1’-isoindole]-3,3’-diones using monobromomalononitrile (MBM) as an efficient organo-brominating agent. Tetrahedron Lett. 2014, 55, 3960–3968.
- 6(a) Yuan, D.; Duan, Z.; Rao, Y.; Ding, M.-W. New efficient synthesis of isoquinoline-1,3(2H,4H)-diones and isoindolin-1-ones via sequential Ugi/cyclization reaction. Tetrahedron 2016, 72, 338–346; (b) Ley, S. V.; Taylor, S. J. A Polymer-Supported [1,3,2] Oxazaphospholidine for the Conversion of Isothiocyanates to Isocyanides and Their Subsequent Use in an Ugi Reaction. Bioorg. Med. Chem. Lett. 2002, 12, 1813–1816; (c) Marandi, F. E.; Yavari, I.; Saeedi, M.; Mahdavi, M.; Shafiee, A. Synthesis of Novel Pyrazino[2,1-a] isoindolediones via Intramolecular Hydroamination of 2,3-Dihydro-3-oxo-2-(prop-2-yn-1- yl)-1H-isoindole-1-carboxamides. Helv. Chim. Acta 2016, 99, 187–190.
- 7 Cho, C. S.; Jiang, L.-H.; Lee, D. Y.; Shim, S. C.; Lee, H. S.; Cho, S. D. Palladium-Catalyzed Synthesis of 3-(Alkylamino)-1-ones by Carbonylative Cyclization of 2-Bromobenzaldehyde with Primary Amines. J. Heterocycl. Chem. 1997, 34, 1371–1374.
- 8(a) Xu, Y.-L.; Zhou, X.-Y.; Chen, L.-Y.; Ma, Y.-F.; Wu, G. The copper-catalyzed radical aminophosphinoylation of maleimides with anilines and diarylphosphine oxides. Org. Chem. Front. 2022, 9, 2471–2476; (b) Huang, J.-P.; Ding, F.; Chen, Z.-Y.; Yang, G.-G.; Wu, J. A multi-component reaction of electron-rich arenes, potassium metabisulfite, aldehydes and aryldiazonium tetrafluoroborates. Org. Chem. Front. 2021, 8, 1461–1465; (c) Ran, C.-K.; Song, L.; Niu, Y.-N.; Wei, M.-K.; Zhang, Z.; Zhou, X.-Y.; Yu, D.-G. Transition-metal-free synthesis of thiazolidine-2-ones and 1,3-thiazinan-2-ones from arylamines, elemental sulfur and CO2. Green Chem. 2021, 23, 274–279; (d) Zhao, P.; Yu, X.-X.; Zhou, Y.; Huang, C.; Wu, Y.-D.; Zhu, Y.-P.; Wu, A.-X. Arylacetylenes as two-carbon synthons: synthesis of eight-membered rings via C≡C bond cleavage. Chem. Commun. 2020, 56, 12554–12557.
- 9(a) Zhu, Y.-M.; Fang, Y.-Z.; Li, H.-Y.; Xu, X.-P.; Ji, S.-J. Divergent Reaction of Isocyanides with o-Bromobenzaldehydes: Synthesis of Ketenimines and Lactams with Isoindolinone Cores. Org. Lett. 2021, 23, 7342−7347; (b) Zhu, Y.-M.; Xu, X.-P.; Ji, S.-J. Divergent Synthesis of Pentacyclic Isoindolinones Enabled by Sequential Insertion of Two Different Isocyanides and Acid Promoted Cyclization of Ketenimines. Org. Lett. 2023, 25, 2041–2046.
- 10(a) Jia, X.-Q.; Zhang, Z.-Y.; Gevorgyan, V. Three-Component Visible-Light-Induced Palladium-Catalyzed 1,2-Alkyl Carbamoylation/ Cyanation of Alkenes. ACS Catal. 2021, 11, 13217–13222; (b) Qiu, G.-Y.; Mamboury, M.; Wang, Q.; Zhu, J.-P. Ketenimines from Isocyanides and Allyl Carbonates: Palladium-Catalyzed Synthesis of β,γ-Unsaturated Amides and Tetrazole. Angew. Chem. Int. Ed. 2016, 55, 15377–15381; (c) Chen, X.-Y.; Qiu, G.-Y.; Liu, R.-Z.; Chen, D.-P.; Chen, Z.-Y. Divergent oriented synthesis (DOS) of aza-heterocyclic amides through palladium-catalyzed ketenimination of 2-iodo-N-(propa-1,2- dien-1-yl)anilines. Org. Chem. Front. 2020, 7, 890–895.
- 11 Zhang, F.-L.; Zhao, R.-H.; Zhu, L.; Yu, Y.-H.; Liao, S.-H.; Wang, Z.-X.; Huang, X.-L. Divergent isoindolinone synthesis through palladium- catalyzed isocyanide bridging C–H activation. Cell Rep. Phys. Sci. 2022, 3, 100776.
- 12(a) Yan, X.; Liao, J.-X.; Lu, Y.-Z.; Liu, J.-S.; Zeng, Y.-L.; Cai, Q. Pd-Catalyzed One-Pot Synthesis of Polysubstituted Acrylamidines from Isocyanides, Diazo Compounds, and Imines. Org. Lett. 2013, 15, 2478–2481; (b) Chen, G.-S.; Chen, S.-J.; Luo, J.; Mao, X.-Y.; Chan, A. S.-C.; Sun, R. W.-Y.; Liu, Y.-L. Tandem Cross-Coupling/Spirocyclization/Mannich-Type Reactions of 3-(2-Isocyanoethyl)indoles with Diazo Compounds toward Polycyclic Spiroindolines. Angew. Chem. Int. Ed. 2020, 59, 614–621; (c) Bestmann, H. J.; Schade, G.; Schmid, G. Synthesis of Cyclic Compounds from Triphenyl(phenyliminovinylidene)phosphorane and Carboxylic Acids. Angew. Chem. Int. Ed. 1980, 19, 822–824.




