Preparation of Optically Active 2,2'-Dibromo-6,6'-diiodo-1,1'-biphenyl: A Powerful Precursor for Modular Synthesis of Functionalized Atropisomers†
Yuanyuan Li
Hefei National Research Center for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
Search for more papers by this authorLonghui Duan
Hefei National Research Center for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
Search for more papers by this authorBiqiong Hong
College of Materials and Chemical Engineering, Minjiang University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorCorresponding Author
Zhenhua Gu
Hefei National Research Center for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
College of Materials and Chemical Engineering, Minjiang University, Fuzhou, Fujian, 350108 China
E-mail: [email protected]Search for more papers by this authorYuanyuan Li
Hefei National Research Center for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
Search for more papers by this authorLonghui Duan
Hefei National Research Center for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
Search for more papers by this authorBiqiong Hong
College of Materials and Chemical Engineering, Minjiang University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorCorresponding Author
Zhenhua Gu
Hefei National Research Center for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui, 230026 China
College of Materials and Chemical Engineering, Minjiang University, Fuzhou, Fujian, 350108 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
The widespread applications of atropisomeric compounds have led to an increasing demand for their synthesis. Rather than synthesizing different functionalized atropisomers individually, an attractive alternative is to identify a key intermediate or precursor that can be readily elaborated and functionalized to realize divergent synthesis of this class of compounds. Building on our previous research on asymmetric ring-opening of cyclic diaryliodoniums, in this work we developed a copper-catalyzed enantioselective ring-opening reaction of ortho,ortho’-dibromo substituted cyclic diaryliodonium with lithium iodide. The resulting optically active product 2,2'-dibromo-6,6'-diiodo-1,1'-biphenyl, possessing two C—Br bonds and two C—I bonds, can be selectively advanced to form different functionalities. Remarkably, the utilities of the product were highlighted by successively demonstrating C—I and C—Br metalation, followed by carboxylation, boroylation, oxygenation, allylation, phosphinylation, etc., all of which provide a new and convenient approach to synthesizing a range of functionalized axially chiral biphenyls.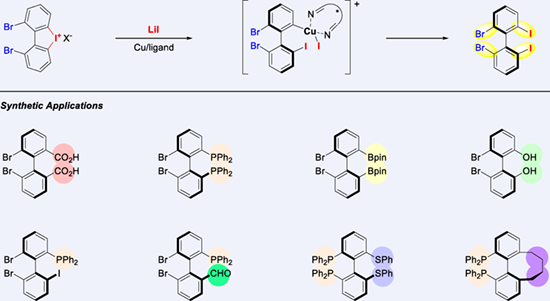
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300438-sup-0001-supinfo.pdfPDF document, 3.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Christie, G. H.; Kenner, J. The Molecular Configurations of Polynuclear Aromatic Compounds. J. Chem. Soc., Trans. 1922, 121, 614–620.
- 2(a) LaPlante, S. R.; Edwards, P. J.; Fader, L. D.; Jakalian, A.; Hucke, O. Revealing Atropisomer Axial Chirality in Drug Discovery. ChemMedChem 2011, 6, 505–513; (b) LaPlante, S. R.; Fader, L. D.; Fandrick, K. R.; Fandrick, D. R.; Hucke, O.; Kemper, R.; Miller, S. P. F.; Edwards, P. J. Assessing Atropisomer Axial Chirality in Drug Discovery and Development. J. Med. Chem. 2011, 54, 7005–7022; (c) Bringmann, G.; Menche, D. Stereoselective Total Synthesis of Axially Chiral Natural Products via Biaryl Lactones. Acc. Chem. Res. 2001, 34, 615–624; (d) Kozlowski, M. C.; Morgan, B. J.; Linton, E. C. Total Synthesis of Chiral Biaryl Natural Products by Asymmetric Biaryl Coupling. Chem. Soc. Rev. 2009, 38, 3193–3207; (e) Smyth, J. E.; Butler, N. M.; Keller, P. A. A Twist of Nature - The Significance of Atropisomers in Biological Systems. Nat. Prod. Rep. 2015, 32, 1562–1583; (f) Toenjes, S. T.; Gustafson, J. L. Atropisomerism in medicinal chemistry: challenges and opportunities. Future Med. Chem. 2018, 10, 409–422; (g) Da, B.-C.; Xiang, S.-H.; Li, S.; Tan, B. Chiral Phosphoric Acid Catalyzed Asymmetric Synthesis of Axially Chiral Compounds. Chin. J. Chem. 2021, 39, 1787–1796; (h) Perreault, S.; Chandrasekhar, J.; Patel, L. Atropisomerism in Drug Discovery: A Medicinal Chemistry Perspective Inspired by Atropisomeric Class I PI3K Inhibitors. Acc. Chem. Res. 2022, 55, 2581–2593.
- 3(a) Noyori, R.; Takaya, H. BINAP: an efficient chiral element for asymmetric catalysis. Acc. Chem. Res. 1990, 23, 345–350; (b) Chen, Y.; Yekta, S.; Yudin, A. K. Modified BINOL Ligands in Asymmetric Catalysis. Chem. Rev. 2003, 103, 3155–3212.
- 4(a) Yoon, T. P.; Jacobsen, E. N. Privileged Chiral Catalysts. Science 2003, 299, 1691–1693;
(b) Privileged Chiral Ligands and Catalysts, Ed.: Q.-L. Zhou, Wiley-VCH Verlag GmbH & Co. KGaA, 2011.
10.1002/9783527635207 Google Scholar
- 5(a) Bringmann, G.; Gulder, T.; Gulder, T. A. M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639; (b) Cheng, J.-K.; Xiang, S.-H.; Li, S.; Ye, L.; Tan, B. Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev. 2021, 121, 4805–4902.
- 6(a) Bringmann, G.; Price Mortimer, A. J.; Keller, P. A.; Gresser, M. J.; Garner, J.; Breuning, M. Atroposelective Synthesis of Axially Chiral Biaryl Compounds. Angew. Chem. Int. Ed. 2005, 44, 5384–5427;
(b) Wencel-Delord, J.; Panossian, A.; Leroux, F. R.; Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 2015, 44, 3418–3430;
(c) Link, A.; Sparr, C. Stereoselective arene formation. Chem. Soc. Rev. 2018, 47, 3804–3815;
(d) Carmona, J. A.; Rodríguez-Franco, C.; Fernández, R.; Hornillos, V.; Las-saletta, J. M. Atroposelective transformation of axially chiral (hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 2021, 50, 2968–2983;
(e) Yao, Q.-J.; Zhang, S.; Zhan, B.-B.; Shi, B.-F. Atroposelective Synthesis of Axially Chiral Biaryls by Palladium-Catalyzed Asymmetric C-H Olefination Enabled by a Transient Chiral Auxiliary. Angew. Chem. Int. Ed. 2017, 56, 6617–6621;
(f) Dong, Y.; Liu, R.; Wang, W. Catalytic asymmetric Catellani-type reaction: A powerful tool for axial chirality construction. Green Synth. Catal. 2020, 1, 83–85;
10.1016/j.gresc.2020.09.002 Google Scholar(g) Liu, C.-X.; Zhang, W.-W.; Yin, S.-Y.; Gu, Q.; You, S.-L. Synthesis of Atropisomers by Transition-Metal-Catalyzed Asymmetric C–H Functionalization Reactions. J. Am. Chem. Soc. 2021, 143, 14025–14040; (h) Da, B.-C.; Xiang, S.-H.; Li, S.; Tan, B. Chiral Phosphoric Acid Catalyzed Asymmetric Synthesis of Axially Chiral Compounds. Chin. J. Chem. 2021, 39, 1787–1796; (i) Zhang, H.-H.; Shi, F. Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications. Acc. Chem. Res. 2022, 55, 2562–2580; (j) Liang, D.; Xiao, W.; Lakhdar, S.; Chen, J. Construction of axially chiral compounds via catalytic asymmetric radical reaction. Green Synth. Catal. 2022, 3, 212–218;10.1016/j.gresc.2022.06.003 Google Scholar(k) Sheng, F.-T.; Yang, S.; Wu, S.-F.; Zhang, Y.-C.; Shi, F. Catalytic Asymmetric Synthesis of Axially Chiral 3,3'-Bisindoles by Direct Coupling of Indole Rings. Chin. J. Chem. 2022, 40, 2151–2160; (l) Cheng, H.-G.; Jia, S.; Zhou, Q. Benzo-Fused-Ring Toolbox Based on Palladium/Norbornene Cooperative Catalysis: Methodology Development and Applications in Natural Product Synthesis. Acc. Chem. Res. 2023, 56, 573–591.
- 7 Kina, A.; Miki, H.; Cho, Y. H.; Hayashi, T. Palladium-Catalyzed Heck and Carbonylation Reactions of a Dinaphthaleneiodonium Salt Forming Functionalized 2-Iodo-1,1’-binaphthyls. Adv. Synth. Catal. 2004, 346, 1728–1732.
- 8 Merritt, E. A.; Olofsson, B. Diaryliodonium salts – from Obscurity to Fame. Angew. Chem. Int. Ed. 2009, 48, 9052–9070.
- 9(a) Zhang, X.; Zhang, K.; Gu, Z. Transition Metal-Catalyzed Biaryl Atropisomer Synthesis via a Torsional Strain Promoted Ring-Opening Reaction. Acc. Chem. Res. 2022, 55, 1620–1633; (b) Zhao, K.; Duan, L. H.; Xu, S. B.; Jiang, J.; Fu, Y.; Gu, Z. Enhanced Reactivity by Torsional Strain of Cyclic Diaryliodonium in Cu-Catalyzed Enantioselective Ring-Opening Reaction. Chem 2018, 4, 599–612; (c) Xu, S. B.; Zhao, K.; Gu, Z. Copper-catalyzed Asymmetric Ring-opening of Cyclic Diaryliodonium with Benzyl and Aliphatic Amines. Adv. Synth. Catal. 2018, 360, 3877–3883; (d) Hou, M.; Deng, R.; Gu, Z. Cu-Catalyzed Enantioselective Atropisomer Synthesis via Thiolative Ring Opening of Five-Membered Cyclic Diaryliodoniums. Org. Lett. 2018, 20, 5779–5783; (e) Xue, X.; Gu, Z. Synthesis of Bridged Biaryl Atropisomers via Sequential Cu- and Pd-Catalyzed Asymmetric Ring Opening and Cyclization. Org. Lett. 2019, 21, 3942–3945; (f) Duan, L.; Zhao, K.; Wang, Z.; Zhang, F. L.; Gu, Z. Enantioselective Ring-Opening/Oxidative Phosphorylation and P-Transfer Reaction of Cyclic Diaryliodoniums. ACS Catal. 2019, 9, 9852–9858; (g) Zhu, K.; Xu, K.; Fang, Q.; Wang, Y.; Tang, B.; Zhang, F.-Z. Enantioselective Synthesis of Axially Chiral Biaryls via Cu-Catalyzed Acyloxylation of Cyclic Diaryliodonium Salts. ACS Catal. 2019, 9, 4951–4957; (h) Cheng, F.; Duan, D.-S.; Jiang, L.-M.; Li, B.-S.; Wang, J.-X.; Zhou, Y.-J.; Jiao, H.-Y.; Wu, T.; Zhu, D.-Y.; Wang, S.-H. Copper-Catalyzed Asymmetric Ring-Opening Reaction of Cyclic Diaryliodonium Salts with Imides. Org. Lett. 2022, 24, 1394–1399; (i) Yang, S.; Zheng, T.; Duan, L.; Xue, X.; Gu, Z. Atroposelective Three- Component Coupling of Cyclic Diaryliodoniums and Sodium Cyanate Enabled by the Dual-Role of Phenol. Angew. Chem. Int. Ed. 2023, 62, e202302749.
- 10(a) Zhu, K.; Song, Z.; Wang, Y.; Zhang, F. Synthesis of 2,2’-Dihalobiaryls via Cu-Catalyzed Halogenation of Cyclic Diaryliodonium Salts. Org. Lett. 2020, 22, 9356–9359; (b) Ke, J.; Zu, B.; Guo, Y.; Li, Y.; He, C. Hexafluoroisopropanol-Enabled Copper-Catalyzed Asymmetric Halogenation of Cyclic Diaryliodoniums for the Synthesis of Axially Chiral 2,2’-Dihalobiaryls. Org. Lett. 2021, 23, 329–333.
- 11 Perron, Q.; Alexakis, A. Catalytic Asymmetric Bromine-Lithium Exchange: A New Tool to Build Axial Chirality. Adv. Synth. Catal. 2010, 352, 2611–2620.
- 12 Ziegler, D. S.; Wei, B.; Knochel, P. Improving the Halogen–Magnesium Exchange by using New Turbo-Grignard Reagents. Chem. - Eur. J. 2019, 25, 2695–2703.
- 13(a) Clary, J. W.; Rettenmaier, T. J.; Snelling, R.; Bryks, W.; Banwell, J.; Wipke, W. T.; Singaram, B. Hydride as a Leaving Group in the Reaction of Pinacolborane with Halides under Ambient Grignard and Barbier Conditions. One-Pot Synthesis of Alkyl, Aryl, Heteroaryl, Vinyl, and Allyl Pinacolboronic Esters. J. Org. Chem. 2011, 76, 9602–9610; (b) Singaram, B.; Murphy, C. L.; Bailey, C. L.; Clary, J. W.; Eagon, S.; Gould, N. Reaction of Grignard Reagents with Diisopropylaminoborane. Synthesis of Alkyl, Aryl, Heteroaryl and Allyl Boronic Acids from Organoc(diisopropyl)aminoborane by a Simple Hydrolysis. Heterocycles 2012, 86, 331–342.
- 14(a) Deng, X.; Guan, Y.-Q.; Huo, N.-N.; Wang, Y.-J.; Lv, H.; Zhang, X.-M. An Efficient and Modular Route to C3*-TunePhos-Type Ligands. Synthesis 2017, 49, 3726–3730; (b) Graff, J.; Debande, T.; Praz, J.; Guénée, L.; Alexakis, A. Asymmetric Bromine–Lithium Exchange: Application toward the Synthesis of Natural Product. Org. Lett. 2013, 15, 4270–4273; (c) Zou, Y.; Geng, H.; Zhang, W.; Yu, S.; Zhang, X. Axially chiral electron-rich TunePhos-type ligand: synthesis and applications in asymmetric hydrogenation. Tetrahedron Lett. 2009, 50, 5777–5779; (d) Kesselgruber, M.; Lotz, M.; Martin, P.; Melone, G.; Müller, M.; Pugin, B.; Naud, F.; Spindler, F.; Thommen, M.; Zbinden, P.; Blaser, H.-U. Solphos: A New Family of Efficient Biaryl Diphosphine Ligands. Chem. Asian J. 2008, 3, 1384–1389.
- 15(a) Tschoerner, M.; Pregosin, P. S.; Albinati, A. Contributions to the Enantioselective Heck Reaction Using MeO−Biphep Ligands. The Case Against Dibenzylidene Acetone. Organometallics 1999, 18, 670–678; (b) Wang, W.-B.; Lu, S.-M.; Yang, P.-Y.; Han, X.-W.; Zhou, Y.-G. Highly Enantioselective Iridium-Catalyzed Hydrogenation of Heteroaromatic Compounds, Quinolines. J. Am. Chem. Soc. 2003, 125, 10536–10537; (c) Genet, J.-P. Asymmetric Catalytic Hydrogenation. Design of New Ru Catalysts and Chiral Ligands: From Laboratory to Industrial Applications. Acc. Chem. Res. 2003, 36, 908–918.
- 16(a) Liu, C.; Lu, X.; Zhang, P.; Yang, H.; Zhu, C.; Fu, H. Axially Chiral Cyclic Diphosphine Ligand-Enabled Palladium-Catalyzed Intramolecular Asymmetric Hydroarylation. iScience 2018, 10, 11–22; (b) Graff, J.; Łastawiecka, E.; Guénée, L.; Leroux, F.; Alexakisa, A. Asymmetric Bromine–Lithium Exchange: Application toward the Synthesis of New Biaryl-Diphosphine Ligands. Adv. Synth. Catal. 2015, 357, 2833–2839; (c) Wan, F.; Tang, W. Phosphorus Ligands from the Zhang Lab: Design, Asymmetric Hydrogenation, and Industrial Applications. Chin. J. Chem. 2021, 39, 954—968.




