Anti-proliferative Properties of Schinensilactone A, A Schinortriterpenoid with 7,8-Seco-1,8-cyclo Scaffold against Caco-2 by Inducing Cell Apoptosis from the Leaves of Schisandra chinensis
Correction(s) for this article
-
Corrigendum
- Volume 41Issue 4Chinese Journal of Chemistry
- pages: 490-490
- First Published online: January 15, 2023
Yan Liu
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
Search for more papers by this authorGuo-Zhen Liu
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
Search for more papers by this authorXiao-Mao Li
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
Search for more papers by this authorPeng Jiang
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
Search for more papers by this authorYong-Gang Xia
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
Search for more papers by this authorCorresponding Author
Hai-Xue Kuang
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Bing-You Yang
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
E-mail: [email protected]; [email protected]Search for more papers by this authorYan Liu
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
Search for more papers by this authorGuo-Zhen Liu
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
Search for more papers by this authorXiao-Mao Li
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
Search for more papers by this authorPeng Jiang
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
Search for more papers by this authorYong-Gang Xia
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
Search for more papers by this authorCorresponding Author
Hai-Xue Kuang
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Bing-You Yang
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, Heilongjiang, 150040 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
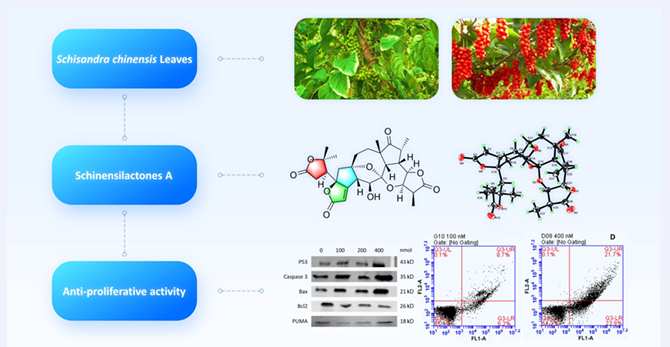
A novel schinortriterpenoid (SNT), schinensilactone A (1), characterized by a unique 7,8-seco-1,8-cyclo-schisanartane scaffold, was isolated from the leaves of Schisandra chinensis (Turcz.) Baill, together with a new SNT (schinensilactone B, 2) and two known (3, 4). Their structures were elucidated using spectroscopy and X-ray diffraction. Furthermore, a hypothetical biosynthetic pathway for 1 was postulated due to its novel carbon skeleton. In addition, 1 exhibited significant anti-proliferative activity against Caco-2 cells originating from five different tumor cell lines, and its preliminary mechanism of action was investigated with respect to the expression of apoptosis-related proteins, including P53, Caspase 3, Bax, PUMA, and Bcl2. These biological activities would further our understanding of the food function of Schisandra chinensis leaves.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200016-sup-0001-Supinfo.pdfPDF document, 3.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Panossian, A.; Wikman, G.; Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail: an overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212.
- 2 Cho, S.; Hong, R.; Yim, P.; Yeom, M.; Lee, B. Schisandra chinensis (Turcz.) Baill, Lycium chinense Mill and Eucommia ulmoides Oliv alleviates disuse muscle atrophy in rats. J. Ethnopharmacol. 2018, 213, 328–339.
- 3 Hancke, J. L.; Burgos, R. A.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. Fitoterapia 1999, 70, 451–471.
- 4 Huang, T. H.; Shen, P. N.; Shen, Y. J. Preparative separation and purification of deoxyschisandrin and γ-schisandrin from Schisandra chinensis (Turcz.) Baill by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1066, 239–242.
- 5 Yang, S. Y.; Yuan, C. H. Schisandra chinensis: A comprehensive review on its phytochemicals and biological activities. Arab. J. Chem. 2021, 14, 103310.
- 6 Zhou, Y.; Men, L. H.; Sun, Y. X.; Wei, M. Y.; Fan, X. Pharmacodynamic effects and molecular mechanisms of lignans from Schisandra chinensis Turcz. (Baill.), a current review. Eur. J. Pharmacol. 2021, 892, 173796.
- 7 Song, J.; Zhou, M.; Zhou, J.; Liang, J. J.; Peng, X. G.; Liu, J. J.; Ruan, H. L. Schincalactones A and B, Two 5/5/6/11/3 Fused Schinortriterpenoids with a 13-Membered Carbon Ring System from Schisandra incarnate. Org. Lett. 2018, 20, 2499–2502.
- 8 Huang, S. X.; Yang, L. B.; Xiao, W. L.; Lei, C.; Liu, J. P.; Lu, Y.; Weng, Z. Y.; Li, L. M.; Li, R. T.; Yu, J. Y.; Zheng, Q. T.; Sun, H. D. Wuweizidilactones A–F: Novel highly oxygenated nortriterpenoids with unusual skeletons isolated from Schisandra chinensis. Chem. - Eur. J. 2007, 13, 4816–4822.
- 9 Huang, S. X.; Li, R. T.; Liu, J. P.; Lu, Y.; Chang, Y.; Lei, C.; Xiao, W. L.; Yang, L. B.; Zheng, Q. T.; Sun, H. D. Isolation and Characterization of Biogenetically Related Highly Oxygenated Nortriterpenoids from Schisandra chinensis. Org. Lett. 2007, 9, 2079–2082.
- 10 Huang, S. X.; Yang, J.; Huang, H.; Li, L. M.; Xiao, W. L. Structural characterization of schintrilactone, a new class of nortriterpenoids from Schisandra chinensis. Org. Lett. 2007, 9, 4175–4178.
- 11 Huang, S. X.; Yang, J.; Huang, H.; Li, L. M.; Xiao, W. L. Isolation and characterization of miscellaneous terpenoids of Schisandra chinensis. Tetrahedron 2008, 19, 4260–4267.
- 12 Huang, S. X.; Han, Q. B.; Lei, C.; Pu, J. X.; Yu, W. L.; Xiao, J. L.; Yang, L. M.; Xu, H. X.; Zheng, Y. T.; Sun, H. D. Schicagenins A-C: Three cagelike nortriterpenoids from leaves and stems of Schisandra chinensis. Org. Lett. 2011, 13, 3848–3851.
- 13 Shi, Y. M.; Wang, L. Y.; Zou, X. S.; Li, X. N.; Shang, S. Z.; Gao, Z. H.; Liang, C. Q.; Luo, H. R.; Li, H. L.; Xiao, W. L.; Sun, H. D. Nortriterpenoids from Schisandra chinensis and their absolute configurational assignments by electronic circular dichroism study. Tetrahedron 2014, 70, 859–868.
- 14 Wang, J. R.; Kurtán, T.; Mándi, A.; Guo, Y. W. Structure and Absolute Stereochemistry of Nortriterpenoids from Schisandra chinensis (Turcz.) Baill. Eur. J. Org. Chem. 2012, 28, 5471–5484.
- 15 Wang, B.; Hu, K.; Li, X. N.; Sun, H. D.; Qin, H. B.; Puno, P. T. Neuroprotective schinortriterpenoids from Schisandra neglecta collected in Medog County, Tibet, China. Bioorg. Chem. 2021, 110, 104785
- 16 Hockenbery, D.; Nuñez, G.; Milliman, C.; Schreiber, R. D.; Korsmeyer, S. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 348, 334–336.
- 17 Tsuchiya, T.; Bonner, H. P.; Engel, T.; Woods, I.; Matsushima, S.; Ward, M. W.; Taki, W.; Henshall, D. C.; Concannon, C. G.; Prehn, J. H. M. Bcl-2 homology domain 3-only proteins Puma and Bim mediate the vulnerability of CA1 hippocampal neurons to proteasome inhibition in vivo. Eur. J. Neurosci. 2011, 33, 401–408.
- 18 Liu, X.; Kim, C. N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of Apoptotic Program in Cell-Free Extracts: Requirement for dATP and Cytochrome. Cell 1996, 86, 147–157.
- 19 Peto, J. Cancer epidemiology in the last century and the next decade. Nature 2001, 411, 390–395.
- 20 Xiao, W. L.; Lei, C.; Ren, J.; Liao, T. G.; Pu, J. X.; Lu, Y.; Pittman, J. C. U.; Zheng, Y. T.; He, F.; Zhu, H. J.; Sun, H. D. Structure elucidation and theoretical investigation of key steps in the biogenetic pathway of schisanartane nortriterpenoids by using DFT method. Chem. - Eur. J. 2010, 14, 11584–11592.
- 21 Xiao, W. L.; Gong, Y. Q.; Wang, R. R.; Weng, Z. Y.; Luo, X.; Li, X. N.; Yang, G. Y.; He, F.; Pu, J. X.; Yang, L. M. Bioactive Nortriterpenoids from Schisandra grandiflora. J. Nat. Prod. 2009, 72, 1678–1681.
- 22 Qin, D.; Shen, W.; Gao, T.; Zuo, S.; Dong, J. Kadanguslactones A-E, further oxygenated terpenoids from Kadsura angustifolia fermented by a symbiotic endophytic fungus, Penicillium ochrochloron SWUKD4.1850. Phytochemistry 2020, 174, 112335.




