[As3M(As3Pb3)]3− (M = Nb, Ta): Ternary Heterometallic Clusters with Early Transition Metal Atoms and Aromatic [Pb3]2−
Cong-Cong Shu
Tianjin Key Lab for Rare Earth Materials and Applications, State Key Laboratory of Elemento-Organic Chemistry, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorLei Qiao
Tianjin Key Lab for Rare Earth Materials and Applications, State Key Laboratory of Elemento-Organic Chemistry, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorCorresponding Author
Alvaro Muñoz-Castro
Grupo de Química Inorgánicay Materiales Moleculares, Facultad de Ingenieria, Universidad Autonoma de Chile, El Llano Subercaseaux, Santiago, 2801 Chile
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zhong-Ming Sun
Tianjin Key Lab for Rare Earth Materials and Applications, State Key Laboratory of Elemento-Organic Chemistry, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCong-Cong Shu
Tianjin Key Lab for Rare Earth Materials and Applications, State Key Laboratory of Elemento-Organic Chemistry, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorLei Qiao
Tianjin Key Lab for Rare Earth Materials and Applications, State Key Laboratory of Elemento-Organic Chemistry, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
Search for more papers by this authorCorresponding Author
Alvaro Muñoz-Castro
Grupo de Química Inorgánicay Materiales Moleculares, Facultad de Ingenieria, Universidad Autonoma de Chile, El Llano Subercaseaux, Santiago, 2801 Chile
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zhong-Ming Sun
Tianjin Key Lab for Rare Earth Materials and Applications, State Key Laboratory of Elemento-Organic Chemistry, School of Materials Science and Engineering, Nankai University, Tianjin, 300350 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMain observation and conclusion
Two ternary clusters, [As3Nb(As3Pb3)]3− 1 and [As3Ta(As3Pb3)]3− 2, were directly extracted from “K8NbPbAs5” and “K8TaPbAs5” intermetallic solid in the presence of ethylenediamine and 2.2.2-crypt, respectively. Both 1 and 2, comprising an electron-poor early transition metal-atom Nb or Ta coordinated to a As3 triangle and a bowl-like As3Pb3, were characterized by single crystal X-ray diffraction, energy dispersive X-ray (EDX) and electrospray ionization mass-spectrometry (ESI-MS). They also represent the first examples of the Pb-As hybrid in solution-based Zintl anion chemistry and DFT calculations revealed a σ-aromaticity of the [Pb3]2− unit.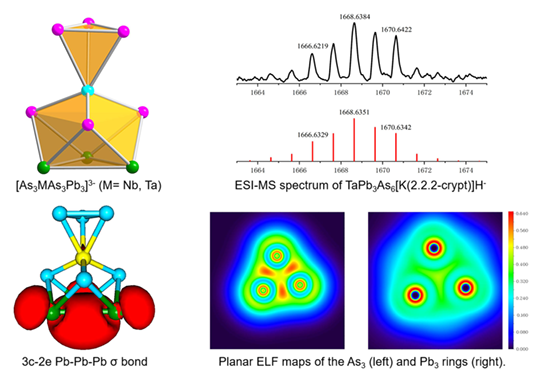
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100161-sup-0001-Supinfo.pdfPDF document, 2.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Wilson, R. J.; Lichtenberger, N.; Weinert, B.; Dehnen, S. Intermetalloid and Heterometallic Clusters Combining p-Block (Semi)Metals with d- or f-Block Metals. Chem. Rev. 2019, 119, 8506−8554.
- 2 Lips, F.; Dehnen, S. [Zn6Sn3Bi8]4−: Expanding the Intermetalloid Zintl Anion Concept to Ternary Systems. Angew. Chem. Int. Ed. 2009, 48, 6435–6438.
- 3 Lips, F.; Schellenberg, I.; Pöttgen, R.; Dehnen, S. The Subtle Influence of Binary versus Homoatomic Zintl Ions: The Phenyl-Ligated Trimetallic Cage [Sn2Sb5(ZnPh)2]3−. Chem.-Eur. J. 2009, 15, 12968–12973.
- 4 Mitzinger, S.; Bandemehr, J.; Reiter, K.; McIndoe, S. J.; Xie, X.; Weigend, F.; Corrigan, J. F.; Dehnen, S. (Ge2P2)2–: A Binary Analogue of P4 as a Precursor to the Ternary Cluster Anion [Cd3(Ge3P)3]3–. Chem. Commun. 2018, 54, 1421–1424.
- 5 Lips, F.; Clérac, R.; Dehnen, S. [Eu@Sn6Bi8]4–: A Mini-Fullerane-Type Zintl Anion Containing a Lanthanide Ion. Angew. Chem. Int. Ed. 2011, 50, 960–964.
- 6 Lips, F.; Hołyńska, M.; Clérac, R.; Linne, U.; Schellenberg, I.; Pöttgen, R.; Florian Weigend, F.; Dehnen, S. Doped Semimetal Clusters: Ternary, Intermetalloid Anions [Ln@Sn7Bi7]4− and [Ln@Sn4Bi9]4− (Ln = La, Ce) with Adjustable Magnetic Properties. J. Am. Chem. Soc. 2012, 134, 1181−1191.
- 7 Gillett-Kunnath, M. M.; Muñoz-Castrob, A.; Sevov, S. C. Tri-metallic deltahedral Zintl ions: experimental and theoretical studies of the novel dimer [(Sn6Ge2Bi)2]4−. Chem. Commun. 2012, 48, 3524−3526.
- 8 Gascoin, F.; Sevov, S. C. Niobium-Arsenic Zintl Phases: A6NbAs5 (A = K, Rb, Cs), K6NbTlAs4, and K8NbPbAs5 with Edge-Bridged Niobium-Centered Tetrahedra of Arsenic, [NbAs4M]n− Where M = As, Tl, Pb. Inorg. Chem. 2002, 41, 2820−2825.
- 9 Mitzinger, S.; Broeckaert, L.; Massa, W.; Weigend, F.; Dehnen, S. [V@Ge8As4]3− and [Nb@Ge8As6]3−: encapsulation of electron-poor transition metal atoms. Chem. Commun. 2015, 51, 3866−3869.
- 10 Mitzinger, S.; Broeckaert, L.; Massa, W.; Weigend, F.; Dehnen, S. Understanding of multimetallic cluster growth. Nat. Commun. 2016, 7, 10480.
- 11 Li, A. M.; Wang, Y.; Downing, D. O.; Chen, F.; Zavalij, P. Y.; Muñoz-Castro, A.; Eichhorn, B. W. Endohedral Plumbaspherenes of the Group 9 Metals: Synthesis, Structure and Properties of the [M@Pb12]3− (M = Co, Rh, Ir) Ions. Chem.-Eur. J. 2020, 26, 5824−5833.
- 12 Shu, C. C.; Morgan, H. W. T.; Qiao, L.; McGrady, J. E.; Sun, Z. M. A family of lead clusters with precious metal cores. Nat. Commun. 2020, 11, 3477.
- 13 Li, A. M.; Wang, Y.; Zavalij, P. Y.; Chen, F.; Muñoz-Castro, A.; Eichhorn, B. W. [Cp*RuPb11]3− and [Cu@Cp*RuPb11]2−: centered and non-centered transition-metal substituted zintl icosahedra. Chem. Commun. 2020, 56, 10859−10862.
- 14 Qiao, L.; Zhang, C.; Shu, C. C.; Morgan, H. W. T.; McGrady, J. E.; Sun, Z. M. [Cu4@E18]4− (E = Sn, Pb): Fused Derivatives of Endohedral Stannaspherene and Plumbaspherene. J. Am. Chem. Soc. 2020, 142, 13288–13293.
- 15 Moses, M. J.; Fettinger, J. C.; Eichhorn, B. W. Interpenetrating As20 Fullerene and Ni12 Icosahedra in the Onion-Skin [As@Ni12@As20]3− Ion. Science 2003, 300, 778.
- 16 Moses, M. J.; Fettinger, J.; Eichhorn, B. Charged Molecular Alloys: Synthesis and Characterization of the Binary Anions Pd7As164− and Pd2As144−. J. Am. Chem. Soc. 2002, 124, 5944–5945.
- 17 Turbervill, R. S. P.; Goicoechea, J. M. Synthesis of 1,2,3-tripnictolide anions by reaction of group 15 Zintl ions with acetylene. Isolation of [E3C2H2]− (E = P, As) and preliminary reactivity studies. Chem. Commun. 2012, 48, 6100–6102.
- 18 Knapp, C. M.; Westcott, B. H.; Raybould, M. A. C.; McGrady, J. E.; Goicoechea, J. M. Transition-metal-mediated activation of the heptaarsenide trianion: isolation of a diaryltetraarsenabutadienediide. Chem. Commun. 2012, 48, 12183–12185.
- 19 Traut, S.; von Hänisch, C. Würfelspiele im Bereich der Heterokubane: Synthese und Charakterisierung von Molekülverbindungen der Systeme Sn/E und Pb/E (E = P/As). Z. Anorg. Allg. Chem. 2011, 637, 1777–1783.
- 20 Pan, F. X.; Xu, C. Q.; Li, L. J.; Min, X. Wang, J. Q.; Li, J.; Zha, H. J.; Sun, Z. M. A niobium-necked cluster [As3Nb(As3Sn3)]3− with aromatic Sn32−. Dalton Trans. 2016, 45, 3874–3879.
- 21 Pyykkö, P. Additive Covalent Radii for Single-, Double-, and Triple- Bonded Molecules and Tetrahedrally Bonded Crystals: A Summary. J. Phys. Chem. A 2015, 119, 2326−2337.
- 22 Hänisch, C. V.; Nikolova, D. [PbAsSiiPr3]6− the First Structurally Characterized Compound with Chemical Bonds between Lead and Arsenic. Z. Anorg. Allg. Chem. 2004, 630, 345−346.
- 23 Kesanli, B.; Fettinger, J.; Scott, B.; Eichhorn, B. Gas Phase, Solution, and Solid State Alkali Ion Binding by the [NbE8]3− (E = As, Sb) Complexes: Synthesis, Structure, and Spectroscopy Bonds between Lead and Arsenic. Inorg. Chem. 2004, 43, 3840–3846.
- 24 Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
- 25 Perdew, J. P., Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 1997, 78, 1396–1396.
- 26 Zubarev, D. Y.; Boldyrev, A. I. Developing paradigms of chemical bonding: adaptive natural density partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207–5217.
- 27 Min, X.; Popov, I. A.; Pan, F. X.; Li, L. J.; Matito, E.; Sun, Z. M.; Wang, L. S.; Boldyrev, A. I. All-Metal Antiaromaticity in Sb4-Type Lanthanocene Anions. Angew. Chem. Int. Ed. 2016, 55, 5531–5535.
- 28 Popov, I. A.; Pan, F. X.; You, X. R.; Li, L. J.; Matito, E.; Liu, C.; Zhai, H. J.; Sun, Z. M.; Boldyrev, A. I. Peculiar All-Metal σ-Aromaticity of the [Au2Sb16]4− Anion in the Solid State. Angew. Chem. Int. Ed. 2016, 55, 15344–15346.
- 29 Popov, I. A.; Starikova, A. A.; Steglenko, D. V.; Boldyrev, A. I. Usefulness of the σ-Aromaticity and σ-Antiaromaticity Concepts for Clusters and Solid-State Compounds. Chem.-Eur. J. 2018, 24, 292.
- 30 Chen, Z. F.; Wannere, C. S.; Corminboeuf, C.; Puchta, R.; von Ragué Schleyer, P. Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chem. Rev. 2005, 105, 3842–3888.
- 31 Wei, J. N.; Zhang, W. X.; Xi, Z. F. The aromatic dianion metalloles. Chem. Sci. 2018, 9, 560–568.
- 32 Gershoni-Poranne, R.; Stanger, A. The NICS-XY-Scan: Identification of Local and Global Ring Currents in Multi-Ring Systems. Chem.-Eur. J. 2014, 20, 5673–5688.
- 33 Rickhaus, M.; Jirasek, M.; Tejerina, L.; Gotfredsen, H.; Peeks, M. D.; Haver, R.; Jiang, H. W.; Claridge, T. D. W.; Anderson, H. L. Global Aromaticity at the Nanoscale. Nat. Chem. 2020, 12, 236–241.
- 34 Heine, T.; Islas, R.; Merino, G. s and p Contributions to the Induced Magnetic Field: Indicators for the Mobility of Electrons in Molecules. J. Comput. Chem. 2007, 28, 302–309.
- 35 Islas, R.; Heine, T.; Merino, G. The Induced Magnetic Field. Acc. Chem. Res. 2012, 45, 215–228.
- 36 Benassi, R.; Lazzeretti, P.; Taddei, F. Magnetic Criteria for Aromaticity. J. Phys. Chem. 1975, 79, 848–851.
- 37 Cuesta, I. G.; De Merás, A. S.; Pelloni, S.; Lazzeretti, P. Understanding the Ring Current Effects on Magnetic Shielding of Hydrogen and Carbon Nuclei in Naphthalene and Anthracene. J. Comput. Chem. 2009, 30, 551–564.
- 38 von Schleyer, P. R.; Jiao, H. What Is Aromaticity? Pure Appl. Chem. 1996, 68, 209–218.
- 39 Liu, C.; Popov, I. A.; Chen, Z.; Boldyrev, A. I.; Sun, Z. M. Aromaticity and Antiaromaticity in Zintl Clusters. Chem.-Eur. J. 2018, 24, 14583–14597.
- 40 Muñoz-Castro, A. The Shielding Cone in Spherical Aromatic Structures: Insights from Models for Spherical 2(N+1)2 Aromatic Fullerenes. Phys. Chem. Chem. Phys. 2017, 19, 12633–12636.




