Photoinduced NaI-Promoted Radical Borylation of Alkyl Halides and Pseudohalides
Chenglan Wang
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310000 China
Search for more papers by this authorLu Zhou
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorKai Yang
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorFeng Zhang
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorCorresponding Author
Qiuling Song
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310000 China
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 China
Institute of Next Generation Matter Transformation, College of Materials Science Engineering at Huaqiao University, 668 Jimei Boulevard, Xiamen, Fujian, 361021 China
E-mail: [email protected]Search for more papers by this authorChenglan Wang
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310000 China
Search for more papers by this authorLu Zhou
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorKai Yang
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorFeng Zhang
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 China
Search for more papers by this authorCorresponding Author
Qiuling Song
Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, Zhejiang, 310000 China
Key Laboratory of Molecule Synthesis and Function Discovery, Fujian Province University, College of Chemistry at Fuzhou University, Fuzhou, Fujian, 350108 China
Institute of Next Generation Matter Transformation, College of Materials Science Engineering at Huaqiao University, 668 Jimei Boulevard, Xiamen, Fujian, 361021 China
E-mail: [email protected]Search for more papers by this authorMain observation and conclusion
A method for photoinduced NaI-promoted radical borylation of aliphatic halides and pseudohalides with bis(catecholato)diboron (B2cat2) as the boron source is introduced. The borylation reaction is operationally simple and shows high functional group tolerance and broad substrate scope. Preliminary mechanistic studies suggest that the reaction proceeds through SN2-based radical-generation strategy.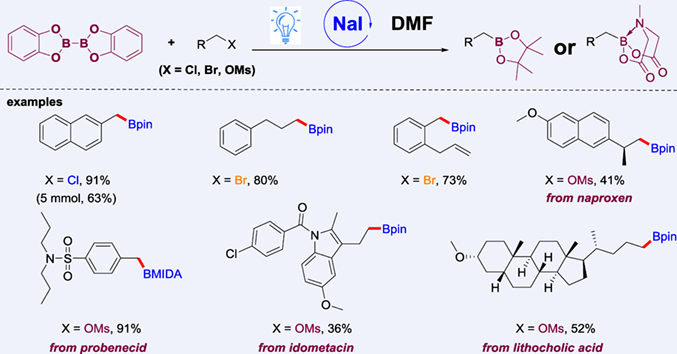
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100115-sup-0001-Supinfo.pdfPDF document, 6.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Hall, D. G. In Boronic Acids, Ed.: Hall, D. G., Wiley−VCH, 2011, pp. 1−133; (b) Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483; (c) Sandford, C.; Aggarwal, V. K. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 2017, 53, 5481–5494; (d) Brooks, W. L. A.; Sumerlin, B. S. Synthesis and Applications of Boronic Acid-Containing Polymers: From Materials to Medicine. Chem. Rev. 2016, 116, 1375–1397.
- 2(a) Fawcett, A.; Pradeilles, J.; Wang, Y.; Mutsuga, T.; Myers, E. L.; Aggarwal, V. K. Photoinduced decarboxylative borylation of carboxylic acids. Science 2017, 357, 283–286; (b) Li, C.; Wang, J.; Barton, L. M.; Yu, S.; Tian, M.; Peters, D. S.; Kumar, M.; Yu, A. W.; Johnson, K. A.; Chatterjee, A. K.; Yan, M.; Baran, P. S. Decarboxylative borylation. Science 2017, 356, eaam7355; (c) Li, J.; Wang, H.; Qiu, Z.; Huang, C.-Y.; Li, C.-J. Metal-Free Direct Deoxygenative Borylation of Aldehydes and Ketones. J. Am. Chem. Soc. 2020, 142, 13011–13020; (d) Larsen, M. A.; Wilson, C. V.; Hartwig, J. F. Iridium-Catalyzed Borylation of Primary Benzylic C–H Bonds without a Directing Group: Scope, Mechanism, and Origins of Selectivity. J. Am. Chem. Soc. 2015, 137, 8633–8643; (e) Candish, L.; Teders, M.; Glorius, F. Transition-Metal- Free, Visible-Light-Enabled Decarboxylative Borylation of Aryl N-Hydroxyphthalimide Esters. J. Am. Chem. Soc. 2017, 139, 7440–7443; (f) Shi, D.; Wang, L.; Xia, C.; Liu, C. Synthesis of Secondary and Tertiary Alkyl Boronic Esters by gem-Carboborylation: Carbonyl Compounds as Bis(electrophile) Equivalents. Angew. Chem. Int. Ed. 2018, 57, 10318–10322; (g) Mlynarski, S. N.; Schuster, C. H.; Morken, J. P. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature 2014, 505, 386–390.
- 3(a) Obligacion, J. V.; Chirik, P. J. Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration. Nat. Rev. Chem. 2018, 2, 15–34; (b) Wen, Y.; Deng, C.; Xie, J.; Kang, X. Recent Synthesis Developments of Organoboron Compounds via Metal-Free Catalytic Borylation of Alkynes and Alkenes. Molecules 2019, 24, 101.
- 4 Brown, H. C.; Cole, T. E. Organoboranes. 31. A simple preparation of boronic esters from organolithium reagents and selected trialkoxyboranes. Organometallics 1983, 2, 1316–1319.
- 5 Lekkala, R.; Lekkala, R.; Moku, B.; Rakesh, K. P.; Qin, H.-L. Recent Developments in Radical-Mediated Transformations of Organohalides. Eur. J. Org. Chem. 2019, 2019, 2769–2806.
- 6(a) Bose, S. K.; Brand, S.; Omoregie, H. O.; Haehnel, M.; Maier, J.; Bringmann, G.; Marder, T. B. Highly Efficient Synthesis of Alkylboronate Esters via Cu(II)-Catalyzed Borylation of Unactivated Alkyl Bromides and Chlorides in Air. ACS Catal. 2016, 6, 8332–8335; (b) Bose, S. K.; Fucke, K.; Liu, L.; Steel, P. G.; Marder, T. B. Zinc-Catalyzed Borylation of Primary, Secondary and Tertiary Alkyl Halides with Alkoxy Diboron Reagents at Room Temperature. Angew. Chem. Int. Ed. 2014, 53, 1799–1803; (c) Yang, C. T.; Zhang, Z. Q.; Tajuddin, H.; Wu, C. C.; Liang, J.; Liu, J. H.; Fu, Y.; Czyzewska, M.; Steel, P. G.; Marder, T. B.; Liu, L. Alkylboronic Esters from Copper-Catalyzed Borylation of Primary and Secondary Alkyl Halides and Pseudohalides. Angew. Chem. Int. Ed. 2012, 51, 528–532.
- 7 Yi, J.; Liu, J. H.; Liang, J.; Dai, J. J.; Yang, C. T.; Fu, Y.; Liu, L. Alkylboronic Esters from Palladium- and Nickel-Catalyzed Borylation of Primary and Secondary Alkyl Bromides. Adv. Synth. Catal. 2012, 354, 1685–1691.
- 8(a) Ito, H.; Kubota, K. Copper(I)-Catalyzed Boryl Substitution of Unactivated Alkyl Halides. Org. Lett. 2012, 14, 890–893; (b) Iwamoto, H.; Endo, K.; Ozawa, Y.; Watanabe, Y.; Kubota, K.; Imamoto, T.; Ito, H. Copper(I)-Catalyzed Enantioconvergent Borylation of Racemic Benzyl Chlorides Enabled by Quadrant-by-Quadrant Structure Modification of Chiral Bisphosphine Ligands. Angew. Chem. Int. Ed. 2019, 58, 11112–11117.
- 9 Dudnik, A. S.; Fu, G. C. Nickel-Catalyzed Coupling Reactions of Alkyl Electrophiles, Including Unactivated Tertiary Halides, to Generate Carbon-Boron Bonds. J. Am. Chem. Soc. 2012, 134, 10693–10697.
- 10(a) Atack, T. C.; Cook, S. P. Manganese-Catalyzed Borylation of Unactivated Alkyl Chlorides. J. Am. Chem. Soc. 2016, 138, 6139–6142; (b) Atack, T. C.; Lecker, R. M.; Cook, S. P. Iron-Catalyzed Borylation of Alkyl Electrophiles. J. Am. Chem. Soc. 2014, 136, 9521–9523.
- 11(a) Cao, Z. C.; Luo, F. X.; Shi, W. J.; Shi, Z. J. Direct borylation of benzyl alcohol and its analogues in the absence of bases. Org. Chem. Front. 2015, 2, 1505–1510; (b) Verma, P. K.; Prasad, K. S.; Varghese, D.; Geetharani, K. Cobalt(I)-Catalyzed Borylation of Unactivated Alkyl Bromides and Chlorides. Org. Lett. 2020, 22, 1431–1436; (c) Yoshida, H.; Takemoto, Y.; Kamio, S.; Osaka, I.; Takaki, K. Copper-catalyzed direct borylation of alkyl, alkenyl and aryl halides with B(dan). Org. Chem. Front. 2017, 4, 1215–1219; (d) Zhao, J. H.; Zhou, Z. Z.; Zhang, Y.; Su, X.; Chen, X. M.; Liang, Y. M. Visible-light-mediated borylation of aryl and alkyl halides with a palladium complex. Org. Biomol. Chem. 2020, 18, 4390–4394.
- 12(a) Nguyen, V. D.; Nguyen, V. T.; Jin, S.; Dang, H. T.; Larionov, O. V. Organoboron chemistry comes to light: Recent advances in photoinduced synthetic approaches to organoboron compounds. Tetrahedron 2019, 75, 584–602. (b) Liu, Q.; Zhang, L.; Mo, F. Organic Borylation Reactions via Radical Mechanism. Acta Chim. Sinica 2020, 78, 1297–1308. (c) Friese, F. W.; Studer, A. New avenues for C–B bond formation via radical intermediates. Chem. Sci. 2019, 10, 8503–8518.
- 13 Cheng, Y.; Mück-Lichtenfeld, C.; Studer, A. Metal-Free Radical Borylation of Alkyl and Aryl Iodides. Angew. Chem. Int. Ed. 2018, 57, 16832–16836.
- 14 Mazzarella, D.; Magagnano, G.; Schweitzer-Chaput, B.; Melchiorre, P. Photochemical Organocatalytic Borylation of Alkyl Chlorides, Bromides, and Sulfonates. ACS Catal. 2019, 9, 5876–5880.
- 15 Liu, Q.; Hong, J.; Sun, B.; Bai, G.; Li, F.; Liu, G.; Yang, Y.; Mo, F. Transition-Metal-Free Borylation of Alkyl Iodides via a Radical Mechanism. Org. Lett. 2019, 21, 6597–6602.
- 16 Zhang, L.; Wu, Z. Q.; Jiao, L. Photoinduced Radical Borylation of Alkyl Bromides Catalyzed by 4-Phenylpyridine. Angew. Chem. Int. Ed. 2020, 59, 2095–2099.
- 17 Cao, Z.-C.; Luo, F.-X.; Shi, W.-J.; Shi, Z.-J. Direct borylation of benzyl alcohol and its analogues in the absence of bases. Org. Chem. Front. 2015, 2, 1505–1510.
- 18 Pitt, W. R.; Parry, D. M.; Perry, B. G.; Groom, C. R. Heteroaromatic Rings of the Future. J. Med. Chem. 2009, 52, 2952–2963.
- 19 Sueki, S.; Kuninobu, Y. Copper-Catalyzed N- and O-Alkylation of Amines and Phenols using Alkylborane Reagents. Org. Lett. 2013, 15, 1544–1547.
- 20 Yang, L.; Tan, D.-H.; Fan, W.-X.; Liu, X.-G.; Wu, J.-Q.; Huang, Z.-S.; Li, Q.; Wang, H. Photochemical Radical C–H Halogenation of Benzyl N-Methyliminodiacetyl (MIDA) Boronates: Synthesis of α-Functionalized Alkyl Boronates. Angew. Chem. Int. Ed. 2021, 60, 3454–3458.




