Catellani Reaction: An Enabling Technology for Vicinal Functionalization of Aryl Halides by Palladium(0)/Norbornene Cooperative Catalysis
Sichan Dong
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, Shaanxi, 710127 China
Search for more papers by this authorCorresponding Author
Xinjun Luan
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, Shaanxi, 710127 China
E-mail: [email protected]Search for more papers by this authorSichan Dong
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, Shaanxi, 710127 China
Search for more papers by this authorCorresponding Author
Xinjun Luan
Key Laboratory of Synthetic and Natural Functional Molecule of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, Shaanxi, 710127 China
E-mail: [email protected]Search for more papers by this authorAbstract
The Catellani reaction, originally discovered by Catellani in 1997, and further developed by Catellani, Lautens and others, has emerged as a powerful strategy for the synthesis of polysubstituted arenes, which would be difficult to access via traditional methods. In this process, both ortho- and ipso-positions of aryl halides could be functionalized simultaneously with different electrophiles and terminating agents under the cooperative catalysis of palladium and norbornene (NBE). This review focuses on the significant progress of such transformations, and the section of typical Catellani reactions is divided into five parts according to the functionalization mode of ortho-C—H bond: alkylation, arylation, amination, acylation or thiolation.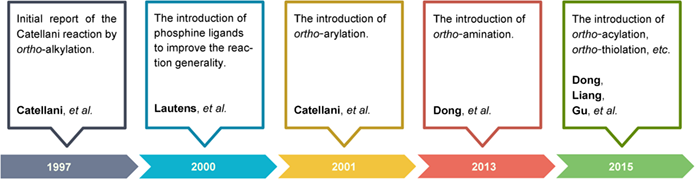
References
- 1 Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859.
- 2 Clarke, E. D.; Spear, L. B.; Mccracken, M. L.; Marques, F. F. C.; Borchers, D. L.; Buckland, S. T.; Ainley, D. G. Validating the Use of Generalized Additive Models and at-Sea Surveys to Estimate Size and Temporal Trends of Seabird Populations. J. Appl. Ecol. 2003, 40, 278c292.
- 3 Theodoridis, G. Chapter 4 Fluorine-Containing Agrochemicals: An Overview of Recent Developments. In Advances in Fluorine Science, Vol. 2, Ed.: Tressaud, A., Elsevier B.V., 2006.
- 4Koizumi, T.-a.; Kanbara, T. Cross-Coupling Polymerization. In Organometallic Reactions and Polymerization, Ed.: Osakada, K., Springer Berlin Heidelberg, Berlin, Heidelberg, 2014.
- 5 Meijere, A. d.; Negishi, E.-I. Handbook of Organopalladium Chemistry for Organic Synthesis, John Wiley & Sons, Inc., 2002.
- 6 Liu, Z.-S.; Gao, Q.; Cheng, H.; Zhou, Q. Alkylating Reagents Employed in Catellani-Type Reactions. Chem.-Eur. J. 2018, 24, 15461–15476.
- 7 Wang, J.; Dong, G. Palladium/Norbornene Cooperative Catalysis. Chem. Rev. 2019, 119, 7478–7528.
- 8 Catellani, M.; Motti, E.; Della Ca’, N. Catalytic Sequential Reactions Involving Palladacycle-Directed Aryl Coupling Steps. Acc. Chem. Res. 2008, 41, 1512–1522.
- 9 Della Ca’, N.; Fontana, M.; Motti, E.; Catellani, M. Pd/Norbornene: A Winning Combination for Selective Aromatic Functionalization via C−H Bond Activation. Acc. Chem. Res. 2016, 49, 1389–1400.
- 10 Yu, J.-Q.; Shi, Z. C−H Activation, Springer Berlin Heidelberg, 2010.
- 11 Ye, J.; Lautens, M. Palladium-Catalysed Norbornene-Mediated C−H Functionalization of Arenes. Nat. Chem. 2015, 7, 863–870.
- 12 Zhao, K.; Ding, L.; Gu, Z. Development of New Electrophiles in Palladium/Norbornene-Catalyzed ortho-Functionalization of Aryl Halides. Synlett 2019, 30, 129−140.
- 13 Li, R.; Dong, G. Structurally Modified Norbornenes: A Key Factor to Modulate Reaction Selectivity in the Palladium/Norbornene Cooperative Catalysis. J. Am. Chem. Soc. 2020, 142, 17859–17875.
- 14 Catellani, M.; Frignani, F.; Rangoni, A. A Complex Catalytic Cycle Leading to a Regioselective Synthesis of o,o'-disubstituted Vinyl- arenes. Angew. Chem. Int. Ed. 1997, 36, 119–122.
- 15 Catellani, M.; Cugini, F. A Catalytic Process Based on Sequential Ortho-Alkylation and Vinylation of Ortho-Alkylaryl Iodides via Palladacycles. Tetrahedron 1999, 55, 6595–6602.
- 16 Wang, J.; Zhou, Y.; Xu, X.; Liu, P.; Dong, G. Entry to 1,2,3,4-Tetrasubstituted Arenes through Addressing the "Meta Constraint" in the Palladium/Norbornene Catalysis. J. Am. Chem. Soc. 2020, 142, 3050–3059.
- 17
Lautens, M.; Piguel, S. A New Route to Fused Aromatic Compounds by Using a Palladium-Catalyzed Alkylation-Alkenylation Sequence. Angew. Chem. Int. Ed. 2000, 39, 1045–1046.
10.1002/(SICI)1521-3773(20000317)39:6<1045::AID-ANIE1045>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 18 Lautens, M.; Paquin, J. F.; Piguel, S.; Dahlmann, M. Palladium-Catalyzed Sequential Alkylation-Alkenylation Reactions and Their Application to the Synthesis of Fused Aromatic Rings. J. Org. Chem. 2001, 66, 8127–8134.
- 19 Alberico, D.; Paquin, J. F.; Lautens, M. Palladium-Catalyzed Sequential Alkylation-Alkenylation Reactions: Application towards the Synthesis of Polyfunctionalized Fused Aromatic Rings. Tetrahedron 2005, 61, 6283–6297.
- 20 Catellani, M.; Motti, E.; Minari, M. Symmetrical and Unsymmetrical 2,6-dialkyl-1,1'-biaryls by Combined Catalysis of Aromatic Alkylation via Palladacycles and Suzuki-type Coupling. Chem. Commun. 2000, 157–158.
- 21 Ding, L.; Sui, X.; Gu, Z. Enantioselective Synthesis of Biaryl Atropisomers via Pd/Norbornene-Catalyzed Three-Component Cross-Couplings. Acs Catal. 2018, 8, 5630–5635.
- 22 Motti, E.; Rossetti, M.; Bocelli, G.; Catellani, M. Palladium Catalyzed Multicomponent Reactions in Ordered Sequence: New Syntheses of o,o'-dialkylsubstituted Diarylacetylenes and Diarylalkylidenehexahydromethanofluorenes. J. Organomet. Chem. 2004, 689, 3741–3749.
- 23 Sun, F.; Li, M.; Gu, Z. Pd/Norbornene-Catalyzed Sequential Ortho-C−H Alkylation and Ipso-Alkynylation: A 1,1-dimethyl-2-alkynol Strategy. Org. Chem. Front. 2016, 3, 309–313.
- 24 Lei, C.; Jin, X.; Zhou, J. Palladium-Catalyzed Alkynylation and Concomitant Ortho Alkylation of Aryl Iodides. Acs Catal. 2016, 6, 1635–1639.
- 25 Bressy, C.; Alberico, D.; Lautens, M. A Route to Annulated Indoles via a Palladium-Catalyzed Tandem Alkylation/Direct Arylation Reaction. J. Am. Chem. Soc. 2005, 127, 13148–13149.
- 26 Mariampillai, B.; Alberico, D.; Bidau, V.; Lautens, M. Synthesis of Polycyclic Benzonitriles via a One-pot Aryl Alkylation/Cyanation Reaction. J. Am. Chem. Soc. 2006, 128, 14436–14437.
- 27 Mariampillai, B.; Alliot, J.; Li, M.; Lautens, M. A Convergent Synthesis of Polysubstituted Aromatic Nitriles via Palladium-Catalyzed C−H Functionalization. J. Am. Chem. Soc. 2007, 129, 15372–15379.
- 28 Thansandote, P.; Raemy, M.; Rudolph, A.; Lautens, M. Synthesis of Benzannulated N-heterocycles by a Palladium-Catalyzed C−C/C−N Coupling of Bromoalkylamines. Org. Lett. 2007, 9, 5255–5258.
- 29 Wilhelm, T.; Lautens, M. Palladium-Catalyzed Alkylation-Hydride Reduction Sequence: Synthesis of Meta-Substituted Arenes. Org. Lett. 2005, 7, 4053–4056.
- 30 Zhou, P.-X.; Zheng, L.; Ma, J.-W.; Ye, Y.-Y.; Liu, X.-Y.; Xu, P.-F.; Liang, Y.-M. Palladium-Catalyzed/Norbornene-Mediated C−H Activation/ N-Tosylhydrazone Insertion Reaction: A Route to Highly Functionalized Vinylarenes. Chem.-Eur. J. 2014, 20, 6745–6751.
- 31 Lei, C.; Jin, X.; Zhou, J. Palladium-Catalyzed Heteroarylation and Concomitant Ortho-Alkylation of Aryl Iodides. Angew. Chem. Int. Ed. 2015, 54, 13397–13400.
- 32 Lei, C.; Cao, J.; Zhou, J. Palladium-Catalyzed Arylation of Ketones and Acetonitrile with Ortho Alkylation of Aryl Rings: De Novo Synthesis of Tetralines and Benzocycloheptenes. Org. Lett. 2016, 18, 6120–6123.
- 33 Pache, S.; Lautens, M. Palladium-Catalyzed Sequential Alkylation- Alkenylation Reactions: New Three-Component Coupling Leading to Oxacycles. Org. Lett. 2003, 5, 4827–4830.
- 34 Wu, X.-X.; Zhou, P.-X.; Wang, L.-J.; Xu, P.-F.; Liang, Y.-M. Synthesis of Polycyclic Substituted Vinylarenes via a One-Pot Intramolecular Aryl Alkylation-N-tosylhydrazone Insertion Reaction. Chem. Commun. 2014, 50, 3882–3884.
- 35 Shen, Y.; Wu, X.-X.; Chen, S.; Xia, Y.; Liang, Y.-M. Synthesis of Polyfluoroarene-Substituted Benzofuran Derivatives via Cooperative Pd/Cu Catalysis. Chem. Commun. 2018, 54, 2256–2259.
- 36 Jiang, W.-T.; Xu, M.-Y.; Yang, S.; Xie, X.-Y.; Xiao, B. Alkylation-Terminated Catellani Reactions Using Alkyl Carbagermatranes. Angew. Chem. Int. Ed. 2020, 59, 20450–20454.
- 37 Martins, A.; Marquardt, U.; Kasravi, N.; Alberico, D.; Lautens, M. Synthesis of Substituted Benzoxacycles via a Domino Ortho-Alkylation/Heck Coupling Sequence. J. Org. Chem. 2006, 71, 4937–4942.
- 38 Thansandote, P.; Chong, E.; Feldmann, K. O.; Lautens, M. Palladium- Catalyzed Domino C−C/C−N Coupling Using a Norbornene Template: Synthesis of Substituted Benzomorpholines, Phenoxazines, and Dihydrodibenzoxazepines. J. Org. Chem. 2010, 75, 3495–3498.
- 39 Gericke, K. M.; Chai, D. I.; Lautens, M. The Versatile Role of Norbornene in C−H Functionalization Processes: Concise Synthesis of Tetracyclic Fused Pyrroles via a Threefold Domino Reaction. Tetrahedron 2008, 64, 6002–6014.
- 40 Aureggi, V.; Davoust, M.; Gericke, K. M.; Lautens, M. A Convenient Synthesis of Indolo- and Pyrrolobenzazepines via a Threefold Norbornene-Mediated Domino Reaction. Synlett 2009, 1004–1008.
- 41 Bai, L.; Liu, J.; Hu, W.; Li, K.; Wang, Y.; Luan, X. Palladium/Norbornene-Catalyzed C−H Alkylation/Alkyne Insertion/Indole Dearomatization Domino Reaction: Assembly of Spiroindolenine-Containing Pentacyclic Frameworks. Angew. Chem. Int. Ed. 2018, 57, 5151–5155.
- 42 Nan, J.; Yuan, Y.; Bai, L.; Liu, J.; Luan, X. Highly Chemoselective Construction of Spiro[4,5]decane-Embedded Polycyclic Scaffolds by a Palladium/Norbornene-Catalyzed C−H Activation/Arene Dearomatization Reaction. Org. Lett. 2018, 20, 7731–7734.
- 43 Mitsudo, K.; Thansandote, P.; Wilhelm, T.; Mariampillai, B.; Lautens, M. Selectively Substituted Thiophenes and Indoles by a Tandem Palladium-Catalyzed Multicomponent Reaction. Org. Lett. 2006, 8, 3939–3942.
- 44 Wang, J.; Dong, Z.; Yang, C.; Dong, G. Modular and Regioselective Synthesis of All-Carbon Tetrasubstituted Olefins Enabled by an Alkenyl Catellani Reaction. Nat. Chem. 2019, 11, 1106–1112.
- 45 Shen, P.-X.; Wang, X. C.; Wang, P.; Zhu, R.-Y.; Yu, J.-Q. Ligand-Enabled Meta-C−H Alkylation and Arylation Using a Modified Norbornene. J. Am. Chem. Soc. 2015, 137, 11574–11577.
- 46 Dong, Z.; Lu, G.; Wang, J.; Liu, P.; Dong, G. Modular Ipso/Ortho Difunctionalization of Aryl Bromides via Palladium/Norbornene Cooperative Catalysis. J. Am. Chem. Soc. 2018, 140, 8551–8562.
- 47 Gao, Q.; Shang, Y.; Song, F.; Ye, J.; Liu, Z. S.; Li, L.; Cheng, H.-G.; Zhou, Q. Modular Dual-Tasked C−H Methylation via the Catellani Strategy. J. Am. Chem. Soc. 2019, 141, 15986–15993.
- 48 Qureshi, Z.; Schlundt, W.; Lautens, M. Introduction of Hindered Electrophiles via C−H Functionalization in a Palladium-Catalyzed Multicomponent Domino Reaction. Synthesis 2015, 47, 2446–2456.
- 49 Lv, W.; Chen, Y.; Wen, S.; Ba, D.; Cheng, G. Modular and Stereoselective Synthesis of C−Aryl Glycosides via Catellani Reaction. J. Am. Chem. Soc. 2020, 142, 14864–14870.
- 50 Ding, Y.-N.; Shi, W.-Y.; Liu, C.; Zheng, N.; Li, M.; An, Y.; Zhang, Z.; Wang, C.-T.; Zhang, B.-S.; Liang, Y.-M. Palladium-Catalyzed ortho-C−H Glycosylation/Ipso-Alkenylation of Aryl Iodides. J. Org. Chem. 2020, 85, 11280–11296.
- 51 Candito, D. A.; Lautens, M. Exploiting the Chemistry of Strained Rings: Synthesis of Indoles via Domino Reaction of Aryl Iodides with 2H-Azirines. Org. Lett. 2010, 12, 3312–3315.
- 52 Liu, C.; Liang, Y.; Zheng, N.; Zhang, B.-S.; Feng, Y.; Bi, S.-W.; Liang, Y.-M. Synthesis of Indolines via a Palladium/Norbornene-Catalyzed Reaction of Aziridines with Aryl Iodides. Chem. Commun. 2018, 54, 3407–3410.
- 53 Li, R.; Liu, F.; Dong, G. Palladium-Catalyzed Asymmetric Annulation between Aryl Iodides and Racemic Epoxides Using a Chiral Norbornene Cocatalyst. Org. Chem. Front. 2018, 5, 3108–3112.
- 54 Cheng, H.-G.; Wu, C.; Chen, H.; Chen, R.; Qian, G.; Geng, Z.; Wei, Q.; Xia, Y.; Zhang, J.; Zhang, Y.; Zhou, Q. Epoxides as Alkylating Reagents for the Catellani Reaction. Angew. Chem. Int. Ed. 2018, 57, 3444–3448.
- 55 Qian, G.; Bai, M.; Gao, S.; Chen, H.; Zhou, S.; Cheng, H.-G.; Yan, W.; Zhou, Q. Modular One-Step Three-Component Synthesis of Tetrahydroisoquinolines Using a Catellani Strategy. Angew. Chem. Int. Ed. 2018, 57, 10980–10984.
- 56 Catellani, M.; Motti, E.; Baratta, S. A Novel Palladium-Catalyzed Synthesis of Phenanthrenes from Ortho-Substituted Aryl Iodides and Diphenyl- or Alkylphenylacetylenes. Org. Lett. 2001, 3, 3611–3614.
- 57 Motti, E.; Ippomei, G.; Deledda, S.; Catellani, M. Synthesis of Selectively Substituted Ortho-Vinylbiphenyls by Palladium-Catalysed Reaction of Ortho-Substituted Aryl Iodides with Olefins. Synthesis- Stuttgart 2003, 2671–2678.
- 58 Catellani, M.; Deledda, S.; Ganchegui, B.; Henin, F.; Motti, E.; Muzart, J. A New Catalytic Method for the Synthesis of Selectively Substituted Biphenyls Containing an Oxoalkyl Chain. J. Organomet. Chem. 2003, 687, 473–482.
- 59 Motti, E.; Mignozzi, A.; Catellani, M. A New Type of Palladium-Catalysed Aromatic Cross-Coupling combined with a Suzuki reaction: Synthesis of Selectively 2,3'-Substituted 1,1';2',1''-Terphenyl Derivatives. J. Mol. Catal. A: Chem. 2003, 204–205, 115–124.
- 60 Deledda, S.; Motti, E.; Catellani, M. Palladium-Catalysed Synthesis of Nonsymmetrically Disubstituted-1,1'-biphenyls from O-Substituted Aryl Iodides through Aryl Coupling and Delayed Hydrogenolysis. Can. J. Chem. 2005, 83, 741–747.
- 61 Maestri, G.; Della Ca’, N.; Catellani, M. A Catalytic Synthesis of Selectively Substituted Biaryls through Sequential Intermolecular Coupling Involving Arene and Ketone C−H Bond Functionalization. Chem. Commun. 2009, 4892–4894.
- 62 Della Ca’, N.; Maestri, G.; Catellani, M. Palladium/Norbornene-Catalyzed Synthesis of Heteroatom-Containing O-Teraryls from Aryl Iodides and Heteroarenes through Double C−H Activation in Sequence. Chem. - Eur. J. 2009, 15, 7850–7853.
- 63 Faccini, F.; Motti, E.; Catellani, M. A New Reaction Sequence Involving Palladium-Catalyzed Unsymmetrical Aryl Coupling. J. Am. Chem. Soc. 2004, 126, 78–79.
- 64 Liu, Z.-S.; Hua, Y.; Gao, Q.; Ma, Y.; Tang, H.; Shang, Y.; Cheng, H.-G.; Zhou, Q. Construction of Axial Chirality via Palladium/Chiral Norbornene Cooperative Catalysis. Nat. Catal. 2020, 3, 727–733.
- 65 Martins, A.; Candito, D. A.; Lautens, M. Palladium-Catalyzed Reductive Ortho-Arylation: Evidence for the Decomposition of 1,2-Dimethoxyethane and Subsequent Arylpalladium(II) Reduction. Org. Lett. 2010, 12, 5186–5188.
- 66 Motti, E.; Della Ca’, N.; Xu, D.; Piersirnoni, A.; Bedogni, E.; Zhou, Z. M.; Catellani, M. A Sequential Pd/Norbornene-Catalyzed Process Generates O-Biaryl Carbaldehydes or Ketones via a Redox Reaction or 6H-Dibenzopyrans by C−O Ring Closure. Org. Lett. 2012, 14, 5792–5795.
- 67 Motti, E.; Della Ca’, N.; Deledda, S.; Fava, E.; Panciroli, F.; Catellani, M. Palladium-Catalyzed Unsymmetrical Aryl Couplings in Sequence Leading to O-teraryls: Dramatic Olefin Effect on Selectivity. Chem. Commun. 2010, 46, 4291–4293.
- 68 Blanchot, M.; Candito, D. A.; Larnaud, F.; Lautens, M. Formal Synthesis of Nitidine and Nk109 via Palladium-Catalyzed Domino Direct Arylation/N-Arylation of Aryl Triflates. Org. Lett. 2011, 13, 1486–1489.
- 69 Ferraccioli, R.; Carenzi, D.; Rombola, O.; Catellani, M. Synthesis of 6-Phenanthridinones and Their Heterocyclic Analogues through Palladium-Catalyzed Sequential Aryl−Aryl and N−aryl Coupling. Org. Lett. 2004, 6, 4759–4762.
- 70 Motti, E.; Faccini, F.; Ferrari, I.; Catellani, M.; Ferraccioli, R. Sequential Unsymmetrical Aryl Coupling of O-Substituted Aryl Iodides with O-Bromophenols and Reaction with Olefins: Palladium-Catalyzed Synthesis of 6H-Dibenzopyran Derivatives. Org. Lett. 2006, 8, 3967–3970.
- 71 Della Ca’, N.; Motti, E.; Catellani, M. Palladium-Catalyzed Synthesis of Selectively Substituted Phenanthridine Derivatives. Adv. Synth. Catal. 2008, 350, 2513–2516.
- 72 Della Ca’, N.; Motti, E.; Mega, A.; Catellani, M. One-Pot Palladium- Catalyzed Synthesis of Selectively Substituted Phenanthridines by Sequential Aryl−Aryl and Heck Couplings, Aza-Michael and Retro- Mannich Reactions. Adv. Synth. Catal. 2010, 352, 1451–1454.
- 73 Candito, D. A.; Lautens, M. Palladium-Catalyzed Domino Direct Arylation/N-Arylation: Convenient Synthesis of Phenanthridines. Angew. Chem. Int. Ed. 2009, 48, 6713–6716.
- 74 Maestri, G.; Larraufie, M. H.; Derat, E.; Ollivier, C.; Fensterbank, L.; Lacote, E.; Malacria, M. Expeditious Synthesis of Phenanthridines from Benzylamines via Dual Palladium Catalysis. Org. Lett. 2010, 12, 5692–5695.
- 75 Sole, D.; Fernandez, I. Controlling the Ambiphilic Nature of Sigma- Arylpalladium Intermediates in Intramolecular Cyclization Reactions. Acc. Chem. Res. 2014, 47, 168–179.
- 76 Zhao, Y.-B.; Mariampillai, B.; Candito, D. A.; Laleu, B.; Li, M.-Z.; Lautens, M. Exploiting the Divergent Reactivity of Aryl-Palladium Intermediates for the Rapid Assembly of Fluorene and Phenanthrene Derivatives. Angew. Chem. Int. Ed. 2009, 48, 1849–1852.
- 77 Yamamoto, Y.; Murayama, T.; Jiang, J.; Yasui, T.; Shibuya, M. The Vinylogous Catellani Reaction: A Combined Computational and Experimental Study. Chem. Sci. 2018, 9, 1191–1199.
- 78 Zhou, H.; Chen, W.; Chen, Z. Pd/Norbornene Collaborative Catalysis on the Divergent Preparation of Heterocyclic Sulfoximine Frameworks. Org. Lett. 2018, 20, 2590–2594.
- 79 Della Ca’, N.; Sassi, G.; Catellani, M. A Direct Palladium-Catalyzed Route to Selectively Substituted Carbazoles through Sequential C−C and C−N Bond Formation: Synthesis of Carbazomycin A. Adv. Synth. Catal. 2008, 350, 2179–2182.
- 80 Motti, E.; Della Ca’, N.; Xu, D.; Armani, S.; Aresta, B. M.; Catellani, M. Competitive Pathways in Pd-catalyzed Synthesis of Arylphenols. Tetrahedron 2013, 69, 4421–4428.
- 81 Zuo, Z.; Wang, H.; Fan, L.; Liu, J.; Wang, Y.; Luan, X. Modular Assembly of Spirocarbocyclic Scaffolds through Pd-0-Catalyzed Intermolecular Dearomatizing [2+2+1] Annulation of Bromonaphthols with Aryl Iodides and Alkynes. Angew. Chem. Int. Ed. 2017, 56, 2767–2771.
- 82 Dong, Z.; Dong, G. Ortho vs Ipso: Site-Selective Pd and Norbornene- Catalyzed Arene C−H Amination Using Aryl Halides. J. Am. Chem. Soc. 2013, 135, 18350–18353.
- 83 Yoo, E. J.; Ma, S.; Mei, T.-S.; Chan, K. S. L.; Yu, J.-Q. Pd-Catalyzed Intermolecular C−H Amination with Alkylamines. J. Am. Chem. Soc. 2011, 133, 7652–7655.
- 84 Shi, H.; Babinski, D. J.; Ritter, T. Modular C−H Functionalization Cascade of Aryl Iodides. J. Am. Chem. Soc. 2015, 137, 3775–3778.
- 85 Chen, Z.-Y.; Ye, C.-Q.; Zhu, H.; Zeng, X.-P.; Yuan, J.-J. Palladium/Norbornene-Mediated Tandem C−H Amination/C−I Alkenylation Reaction of Aryl Iodides with Secondary Cyclic O-Benzoyl Hydroxylamines and Activated Terminal Olefins. Chem. - Eur. J. 2014, 20, 4237–4241.
- 86 Wang, J.; Gu, Z. Synthesis of 2-(1-Alkoxyvinyl)anilines by Palladium/ Norbornene-Catalyzed Amination Followed by Termination with Vinyl Ethers. Adv. Synth. Catal. 2016, 358, 2990–2995.
- 87 Ye, C.-Q.; Zhu, H.; Chen, Z.-Y. Synthesis of Biaryl Tertiary Amines through Pd/Norbornene Joint Catalysis in a Remote C−H Amination/ Suzuki Coupling Reaction. J. Org. Chem. 2014, 79, 8900–8905.
- 88 Wilson, J. E. Palladium-Catalyzed Catellani-type Couplings Using Methylating Reagents for the Synthesis of Highly Substituted Ortho- Methyl-Arenes and Heteroarenes. Tetrahedron Lett. 2016, 57, 5053–5056.
- 89 Zhou, P.-X.; Ye, Y.-Y.; Ma, J.-W.; Zheng, L.; Tang, Q.; Qiu, Y.-F.; Song, B.; Qiu, Z.-H.; Xu, P.-F.; Liang, Y.-M. Palladium-Catalyzed/Norbornene-Mediated Ortho-Amination/N-Tosylhydrazone Insertion on Reaction: An Approach to the Synthesis of Ortho-Aminated Vinylarenes. J. Org. Chem. 2014, 79, 6627–6633.
- 90 Sun, F.; Gu, Z. Decarboxylative Alkynyl Termination of Palladium- Catalyzed Catellani Reaction: A Facile Synthesis of α-Alkynyl Anilines via Ortho C−H Amination and Alkynylation. Org. Lett. 2015, 17, 2222–2225.
- 91 Pan, S.; Ma, X.; Zhong, D.; Chen, W.; Liu, M.; Wu, H. Palladium-Catalyzed One-Pot Consecutive Amination and Sonogashira Coupling for Selective Synthesis of 2-Alkynylanilines. Adv. Synth. Catal. 2015, 357, 3052–3056.
- 92 Luo, B.; Gao, J. M.; Lautens, M. Palladium-Catalyzed Norbornene- Mediated Tandem Amination/Cyanation Reaction: A Method for the Synthesis of ortho-Aminated Benzonitriles. Org. Lett. 2016, 18, 4166–4169.
- 93 Majhi, B.; Ranu, B. C. Palladium-Catalyzed Norbornene-Mediated Tandem Ortho-C−H-Amination/Ipso-C−I-Cyanation of Iodoarenes: Regiospecific Synthesis of 2-Aminobenzonitrile. Org. Lett. 2016, 18, 4162–4165.
- 94 Fu, W. C.; Zheng, B.; Zhao, Q. Y.; Chan, W. T. K.; Kwong, F. Y. Cascade Amination and Acetone Monoarylation with Aryl Iodides by Palladium/Norbornene Cooperative Catalysis. Org. Lett. 2017, 19, 4335–4338.
- 95 Fan, L.; Liu, J.; Bai, L.; Wang, Y.; Luan, X. Rapid Assembly of Diversely Functionalized Spiroindenes by a Three-Component Palladium-Catalyzed C−H Amination/Phenol Dearomatization Domino Reaction. Angew. Chem. Int. Ed. 2017, 56, 14257–14261.
- 96 Zhang, B.-S.; Li, Y.; Zhang, Z.; An, Y.; Wen, Y.-H.; Gou, X.-Y.; Quan, S.-Q.; Wang, X.-G.; Liang, Y.-M. Synthesis of C4-Aminated Indoles via a Catellani and Retro-Diels−Alder Strategy. J. Am. Chem. Soc. 2019, 141, 9731–9738.
- 97 Whyte, A.; Olson, M. E.; Lautens, M. Palladium-Catalyzed, Norbornene-Mediated, Ortho-Amination Ipso-Amidation: Sequential C−N Bond Formation. Org. Lett. 2018, 20, 345–348.
- 98 Zhang, B.-S.; Li, Y.-K.; An, Y.; Zhang, Z.; Liu, C.; Wang, X.-G.; Liang, Y.-M. Carboxylate Ligand-Exchanged Amination/C(sp3)−H Arylation Reaction via Pd/Norbornene Cooperative Catalysis. ACS Catal. 2018, 8, 11827–11833.
- 99 An, Y.; Zhang, B.-S.; Zhang, Z.; Liu, C.; Gou, X.-Y.; Ding, Y.-N.; Liang, Y.-M. A Carboxylate-Assisted Amination/Unactivated C(sp2)−H Arylation Reaction via a Palladium/Norbornene Cooperative Catalysis. Chem. Commun. 2020, 56, 5933–5936.
- 100 Zhang, B.-S.; Wang, F.; Yang, Y.-H.; Gou, X.-Y.; Qiu, Y.-F.; Wang, X.-C.; Liang, Y.-M.; Li, Y.; Quan, Z.-J. Synthesis of C4-Substituted Indoles via a Catellani and C−N Bond Activation Strategy. Org. Lett. 2020, 22, 8267–8271.
- 101 Zhou, P.-X.; Ye, Y.-Y.; Liu, C.; Zhao, L.-B.; Hou, J.-Y.; Chen, D.-Q.; Tang, Q.; Wang, A.-Q.; Zhang, J.-Y.; Huang, Q.-X.; Xu, P.-F.; Liang, Y.-M. Palladium-Catalyzed Acylation/Alkenylation of Aryl Iodide: A Domino Approach Based on the Catellani-Lautens Reaction. ACS Catal. 2015, 5, 4927–4931.
- 102 Dong, Z.; Wang, J.; Ren, Z.; Dong, G. Ortho C−H Acylation of Aryl Iodides by Palladium/Norbornene Catalysis. Angew. Chem. Int. Ed. 2015, 54, 12664–12668.
- 103 Huang, Y.; Zhu, R.; Zhao, K.; Gu, Z. Palladium-Catalyzed Catellani Ortho-Acylation Reaction: An Efficient and Regiospecific Synthesis of Diaryl Ketones. Angew. Chem. Int. Ed. 2015, 54, 12669–12672.
- 104 Pan, S.; Wu, F.; Yu, R.; Chen, W. Palladium-Catalyzed Sequential Acylation/Cyanation of Aryl Iodides: A Regiospecific Synthesis of 2-Cyanoaryl Ketones. J. Org. Chem. 2016, 81, 1558–1564.
- 105 Yu, S.-P.; Zhong, Y.; Gu, T.; Wu, W.-Y.; Fan, T.-Y.; Li, N.-G.; Shi, Z.-H.; Tang, Y.-P.; Duan, J.-A. Palladium-Catalyzed One-Pot Catellani Reaction: An Efficient Synthesis of Alpha-Alkynyl Aromatic Ketones via Ortho Acylation and Ipso Alkynylation. Tetrahedron 2018, 74, 5942–5949.
- 106 Zhang, P.; Pan, S.; Chen, W.; Liu, M.; Wu, H. Palladium-Catalyzed Sequential Heteroarylation/Acylation Reactions of lodobenzenes: Synthesis of Functionalized Benzo[d]oxazoles. J. Org. Chem. 2018, 83, 3354–3360.
- 107 Liu, F.; Dong, Z.; Wang, J.; Dong, G. Palladium/Norbornene-Catalyzed Indenone Synthesis from Simple Aryl Iodides: Concise Syntheses of PauciflorolF and AcredinoneA. Angew. Chem. Int. Ed. 2019, 58, 2144–2148.
- 108 Prokopcová, H.; Kappe, C. O. The Liebeskind-Srogl C−C Cross-Coupling Reaction. Angew. Chem. Int. Ed. 2009, 48, 2276–2286.
- 109 Sun, F.; Li, M.; He, C.; Wang, B.; Li, B.; Sui, X.; Gu, Z. Cleavage of the C(O)−S Bond of Thioesters by Palladium/Norbornene/Copper Cooperative Catalysis: An Efficient Synthesis of 2-(Arylthio)aryl Ketones. J. Am. Chem. Soc. 2016, 138, 7456–7459.
- 110 Fan, X.; Gu, Z. Palladium/Norbornene-Catalyzed Ortho-Acylation and Ipso-Selenation via C(O)−Se Bond Cleavage: Synthesis of α-Carbonyl Selane. Org. Lett. 2018, 20, 1187–1190.
- 111 Feng, Y.; Wang, Y.; Zhao, S.; Zhang, D.-P.; Li, X.; Liu, H.; Dong, Y.; Sun, F.-G. A Practical Ortho-Acylation of Aryl Iodides Enabled by Moisture- Insensitive Activated Esters via Palladium/Norbornene Catalysis. Org. Chem. Front. 2020, 7, 3420–3426.
- 112 Wang, J.; Zhang, L.; Dong, Z.; Dong, G. Reagent-Enabled Ortho- Alkoxycarbonylation of Aryl Iodides via Palladium/Norbornene Catalysis. Chem 2016, 1, 581–591.
- 113 Li, X.; Pan, J.; Song, S.; Jiao, N. Pd-Catalyzed Dehydrogenative Annulation Approach for the Efficient Synthesis of Phenanthridinones. Chem. Sci. 2016, 7, 5384–5389.
- 114 Chen, C.; Liu, L.; Sun, W.; Ding, J.; Zhu, Y.-P.; Zhu, B. Pd/Nbe-Catalyzed Sequential Carbamoylation/Olefination of Aryl Iodides. Org. Chem. Front. 2020, 7, 3179–3185.
- 115 Cai, W.; Gu, Z. Selective Ortho Thiolation Enabled by Tuning the Ancillary Ligand in Palladium/Norbornene Catalysis. Org. Lett. 2019, 21, 3204–3209.
- 116 Li, R.; Zhou, Y.; Yoon, K.-Y.; Dong, Z.; Dong, G. Sulfenamide-Enabled Ortho Thiolation of Aryl Iodides via Palladium/Norbornene Cooperative Catalysis. Nat. Commun. 2019, 10, 3555.
- 117 Wang, J.; Li, R.; Dong, Z.; Liu, P.; Dong, G. Complementary Site- Selectivity in Arene Functionalization Enabled by Overcoming the Ortho Constraint in Palladium/Norbornene Catalysis. Nat. Chem. 2018, 10, 866–872.
- 118 Wang, J.; Qin, C.; Lumb, J.-P.; Luan, X. Regioselective Synthesis of Polyfunctional Arenes by a 4-Component Catellani Reaction. Chem 2020, 6, 2097–2109.
- 119 Rago, A. J.; Dong, G. Unexpected Ortho-Heck Reaction under the Catellani Conditions. Org. Lett. 2020, 22, 3770–3774.
- 120 Sui, X.; Zhu, R.; Li, G.; Ma, X.; Gu, Z. Pd-Catalyzed Chemoselective Catellani ortho-Arylation of Iodopyrroles: Rapid Total Synthesis of Rhazinal. J. Am. Chem. Soc. 2013, 135, 9318–9321.
- 121 Weinstabl, H.; Suhartono, M.; Qureshi, Z.; Lautens, M. Total Synthesis of (+)-Linoxepin by Utilizing the Catellani Reaction. Angew. Chem. Int. Ed. 2013, 52, 5305–5308.
- 122 Yoon, K.-Y.; Dong, G. Modular In Situ Functionalization Strategy: Multicomponent Polymerization by Palladium/Norbornene Cooperative Catalysis. Angew. Chem. Int. Ed. 2018, 57, 8592−8596.
- 123 Yoon, K.-Y.; Xue, Y.; Dong, G. Three-Step Synthesis of A Less Aggregated Water-Soluble Poly(Para-Phenylene Ethynylene) with Meta- Side-Chains by Palladium/Norbornene Cooperative Catalysis. Macromolecules 2019, 52, 1663–1670.
- 124 Kim, D.-S.; Park, W.-J.; Jun, C.-H. Metal−Organic Cooperative Catalysis in C−H and C−C Bond Activation. Chem. Rev. 2017, 117, 8977–9015.
- 125 Cheng, H.; Chen, S.; Chen, R.; Zhou, Q. Palladium(II)-Initiated Catellani-Type Reactions. Angew. Chem. Int. Ed. 2019, 58, 5832−5844.




