Feng Ligand: Privileged Chiral Ligand in Asymmetric Catalysis
Ming-Yang Wang
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310027 China
Search for more papers by this authorCorresponding Author
Wei Li
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310027 China
E-mail: [email protected]Search for more papers by this authorMing-Yang Wang
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310027 China
Search for more papers by this authorCorresponding Author
Wei Li
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310027 China
E-mail: [email protected]Search for more papers by this authorAbstract
Catalysts and ligands possessing the great ability to tolerate over a wide range of mechanistically unrelated reactions are remarked as "privileged", which are rather scarce but extremely meaningful in asymmetric catalysis. Feng and co-workers have developed a library of conformationally flexible, C2-symmetric N,N'-dioxide amide compounds with original design and featured structure (named as Feng ligand now). They were initially reported as chiral organocatalysts in 2005 and have been further developed as a new class of privileged chiral ligands since 2006. Tremendous success, including versatile coordination chemistry with plenty of metal sources (main-group metals, transition metals, and rare-earth metals), a truly broad scope of asymmetric reactions (more than 50 types), diverse areas of catalysis (organocatalysis, Lewis-acid catalysis, bimetallic relay catalysis, and photocatalysis), numerous synthetic applications of bioactive compounds, has been achieved using Feng N,N'-dioxide. Besides, they demonstrate that chiral ligands with conformationally flexible property can offer excellent chiral environment as well, which challenges the conventional idea preferring rigid structures in the design of chiral ligands. Herein, we briefly introduced the discovery of Feng ligand and the millstones during the development. We also covered the successful applications of Feng ligand by other scientists as well as novel chiral ligands inspired by them.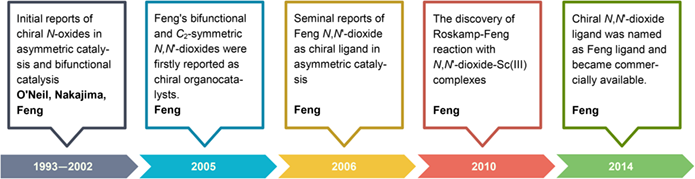
References
- 1 Jacobsen, E. N. Comprehensive Asymmetric Catalysis, Springer, New York, 1999.
- 2 Blaser, H.-U.; Federsel, H.-J. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions, 2nd ed., Wiley-VCH, 2010.
- 3(a) Yoon, T. P.; Jacobsen, E. N. Privileged Chiral Catalysts. Science 2003, 299, 1691–1693;
(b) Zhou, Q.-L. Privileged Chiral Ligands and Catalysts, Wiley-VCH, Weinheim, 2011;
10.1002/9783527635207 Google Scholar(c) Mikami, K. Chiral Lewis Acids. In Topics in Organometallic Chemistry, Springer, Switzerland, 2018.10.1007/978-3-319-70806-5 Google Scholar
- 4(a) Liu, X. H.; Lin, L. L.; Feng, X. M. Chiral N,N'-Dioxides: New Ligands and Organocatalysts for Catalytic Asymmetric Reactions. Acc. Chem. Res. 2011, 44, 574–587; (b) Liu, X. H.; Lin, L. L.; Feng, X. M. Chiral N,N'-Dioxide Ligands: Synthesis, Coordination Chemistry and Asymmetric Catalysis. Org. Chem. Front. 2014, 1, 298–302; (c) Liu, X. H.; Dong, S. X.; Lin, L. L.; Feng, X. M. Chiral Amino Acids-Derived Catalysts and Ligands. Chin. J. Chem. 2018, 36, 791–797; (d) Wang, Z.; Liu, X. H.; Feng, X. M. Asymmetric Catalysis Enabled by Chiral N,N'-Dioxide-Nickel(II) Complexes. Aldrichim. ACTA 2020, 53, 3–10; (e) Cao, W. D.; Liu, X. H.; Feng, X. M. Chiral N,N′-Dioxide and Their Metal Complexes in Asymmetric Catalysis. Chin. Sci. Bull. 2020, 65, 2941–2951.
- 5 Karayannis, N. M.; Pytlewski, L. L.; Mikulski, C. M. Metal Complexes of Aromatic Amine N-Oxides. Coord. Chem. Rev. 1973, 11, 93–159.
- 6 O'Neil, I. A.; Miller, N. D.; Peake, J.; Barkley, J. V.; Low, C. M. R.; Kalindjian, S. B. The Novel Use of Proline Derived Amine Oxides in Controlling Amide Conformation. Synlett 1993, 515–518.
- 7 O'Neil, I. A.; Turner, C. D.; Kalindjian, S. B. Homochiral Amine Oxides in the Enantioselective Reduction of Ketones. Synlett 1997, 777–780.
- 8 Nakajima, M.; Sasaki, Y.; Shiro, M.; Hashimoto, S. A Novel Axially Dissymmetric Chiral Ligand Based on Amine N-Oxide: (R)- and (S)-3,3'-Dimethyl-2,2'-biquinoline N,N'-Dioxide. Tetrahedron: Asymmetry 1997, 8, 341–344.
- 9 Nakajima, M.; Saito, M.; Shiro, M.; Hashimoto, S. (S)-3,3'-Dimethyl- 2,2'-biquinoline N,N'-Dioxide as an Efficient Catalyst for Enantioselective Addition of Allyltrichlorosilanes to Aldehydes. J. Am. Chem. Soc. 1998, 120, 6419–6420.
- 10 Nakajima, M. Chiral N-Oxides as Catalysts or Ligands in Enantioselective Reactions. J. Synth. Org. Chem. Jpn. 2003, 61, 1081–1087.
- 11(a) Chelucci, G.; Murineddu, G.; Pinna, G. A. Chiral Pyridine N-Oxides: Useful Ligands for Asymmetric Catalysis. Tetrahedron: Asymmetry 2004, 15, 1373–1389; (b) Malkov, A. V.; Kocovsky, P. Chiral N-Oxides in Asymmetric Catalysis. Eur. J. Org. Chem. 2007, 29–36.
- 12 Liu, B.; Feng, X. M.; Chen, F. X.; Zhang, G. L.; Cui, X.; Jiang, Y. Z. Enantioselective Strecker Reaction Promoted by Chiral N-Oxides. Synlett 2001, 1551–1554.
- 13(a) Kanai, M.; Hamashima, Y.; Shibasaki, M. Design of a New Bifunctional Asymmetric Catalyst from Carbohydrates: Application to Catalytic Asymmetric Cyanosilylation of Aldehydes and Acetophenone. Tetrahedron Lett. 2000, 41, 2405–2409; (b) Sawada, D.; Kanai, M.; Shibasaki, M. Enantioselective Total Synthesis of Epothilones A and B Using Multifunctional Asymmetric Catalysis. J. Am. Chem. Soc. 2000, 122, 10521–10532.
- 14 Shen, Y. C.; Feng, X. M.; Zhang, G. L.; Jiang, Y. Z. Asymmetric Cyanosilylation of Ketones Catalyzed by Chiral N-Oxide-Titanium Complex. Synlett 2002, 1353–1355.
- 15 Wen, Y. H.; Huang, X.; Huang, J. L.; Xiong, Y.; Qin, B.; Feng, X. M. Asymmetric Cyanosilylation of Aldehydes Catalyzed by Novel Organocatalysts. Synlett 2005, 2445–2448.
- 16(a) Qin, B.; Liu, X. H.; Shi, J.; Zheng, K.; Zhao, H. T.; Feng, X. M. Enantioselective Cyanosilylation of α,α-Dialkoxy Ketones Catalyzed by Proline-Derived in situ Prepared N-Oxides as Bifunctional Organocatalysts. J. Org. Chem. 2007, 72, 2374–2378; (b) Huang, J. L.; Liu, X. H.; Wen, Y. H.; Qin, B.; Feng, X. M. Enantioselective Strecker Reaction of Phosphinoyl Ketoimines Catalyzed by in situ Prepared Chiral N,N'-Dioxides. J. Org. Chem. 2007, 72, 204–208; (c) Cai, Y. F.; Wang, W. T.; Shen, K.; Wang, J.; Hu, X. L.; Lin, L. L.; Liu, X. H.; Feng, X. M. Highly Enantioselective α-Chlorination of Cyclic β-Ketoesters Catalyzed by N,N'-Dioxide Using NCS as the Chlorine Source. Chem. Commun. 2010, 46, 1250–1252.
- 17 Li, Q. H.; Liu, X. H.; Wang, J.; Shen, K.; Feng, X. M. Asymmetric Cyanosilylation of Ketones Catalyzed by Novel Chiral N,N'-Dioxide Titanium Complexes. Tetrahedron Lett. 2006, 47, 4011–4014.
- 18 Zhang, X.; Chen, D. H.; Liu, X. H.; Feng, X. M. Enantioselective Allylation of Ketones Catalyzed by N,N'-Dioxide and Indium(III) Complex. J. Org. Chem. 2007, 72, 5227–5233.
- 19 Liu, X. H.; Zheng, H. F.; Xia, Y.; Lin, L. L.; Feng, X. M. Asymmetric Cycloaddition and Cyclization Reactions Catalyzed by Chiral N,N'-Dioxide-Metal Complexes. Acc. Chem. Res. 2017, 50, 2621–2631.
- 20 Zheng, K.; Liu, X. H.; Feng, X. M. Recent Advances in Metal-Catalyzed Asymmetric 1,4-Conjugate Addition (ACA) of Nonorganometallic Nucleophiles. Chem. Rev. 2018, 118, 7586–7656.
- 21 Bakhtiari, A.; Safaei-Ghomi, J. Effects of Chiral Ligands on the Asymmetric Carbonyl-Ene Reaction. Synlett 2019, 30, 1738–1764.
- 22(a) Wang, F.; Liu, X. H.; Zhang, Y. L.; Lin, L. L.; Feng, X. M. Highly Enantioselective Synthesis of Tertiary Alcohols: C2-Symmetric N,N'-Dioxide- Sc(III) Complex Promoted Direct Aldol Reaction of α-Ketoesters and Diazoacetate Esters. Chem. Commun. 2009, 7297–7299; (b) Shen, K.; Liu, X. H.; Zheng, K.; Li, W.; Hu, X. L.; Lin, L. L.; Feng, X. M. Catalytic Asymmetric Synthesis of 3-(α-Hydroxy-β-carbonyl)-oxindoles by a ScIII-Catalyzed Direct Aldol-Type Reaction. Chem. Eur. J. 2010, 16, 3736–3742.
- 23(a) Li, X.; Liu, X. H.; Fu, Y.; Wang, L.; Zhou, L.; Feng, X. M. Direct Allylation of Aldimines Catalyzed by C2-Symmetric N,N'-Dioxide-ScIII Complexes: Highly Enantioselective Synthesis of Homoallylic Amines. Chem. Eur. J. 2008, 14, 4796–4098; (b) Zhou, L.; Lin, L. L.; Ji, J.; Xie, M. S.; Liu, X. H.; Feng, X. M. Catalytic Asymmetric Vinylogous Mannich- type (AVM) Reaction of Nonactivated α-Angelica Lactone. Org. Lett. 2011, 13, 3056–3059; (c) Zhao, J. N.; Liu, X. H.; Luo, W. W.; Xie, M. S.; Lin, L. L.; Feng, X. M. Asymmetric Synthesis of β-Amino Nitriles through a ScIII-Catalyzed Three-Component Mannich Reaction of Silyl Ketene Imines. Angew. Chem. Int. Ed. 2013, 52, 3473–3477.
- 24 Zhang, Y.; Liu, X. H.; Lin, L. L.; Feng, X. M. Recent Advance in Catalytic Asymmetric Friedel-Crafts Reactions. Prog. Chem. 2018, 30, 491–504.
- 25 Yu, Z. P.; Liu, X. H.; Dong, Z. H.; Xie, M. S.; Feng, X. M. An N,N'-Dioxide/ In(OTf)3 Catalyst for the Asymmetric Hetero-Diels-Alder Reaction between Danishefsky's Dienes and Aldehydes: Application in the Total Synthesis of Triketide. Angew. Chem. Int. Ed. 2008, 47, 1308–1311.
- 26 Xie, M. S.; Liu, X. H.; Wu, X. X.; Cai, Y. F.; Lin, L. L.; Feng, X. M. Catalytic Asymmetric [8+2] Cycloaddition: Synthesis of Cycloheptatriene- Fused Pyrrole Derivatives. Angew. Chem. Int. Ed. 2013, 52, 5604–5607.
- 27(a) Wang, Z.; Chen, Z. L.; Bai, S.; Li, W.; Liu, X. H.; Lin, L. L.; Feng, X. M. Highly Z-Selective Asymmetric Conjugate Addition of Alkynones with Pyrazol-5-ones Promoted by N,N'-Dioxide-Metal Complexes. Angew. Chem. Int. Ed. 2012, 51, 2776–2779; (b) Ge, S. L.; Kang, T. F.; Lin, L. L.; Zhang, X. Y.; Zhao, P.; Liu, X. H.; Feng, X. M. Chiral N,N'-Dioxide/ Sc(OTf)3 Complex-Catalyzed Asymmetric Dearomatization of β-Naphthols. Chem. Commun. 2017, 53, 11759–11762; (c) Ji, J.; Lin, L. L.; Tang, Q.; Kang, T. F.; Liu, X. H.; Feng, X. M. Highly Efficient Asymmetric Synthesis of Chiral γ-Alkenyl Butenolides Catalyzed by Chiral N,N'-Dioxide-Scandium(III) Complexes. ACS Catal. 2017, 7, 3763–3767.
- 28 Hui, Y. H.; Jiang, J.; Wang, W. T.; Chen, W. L.; Cai, Y. F.; Lin, L. L.; Liu, X. H.; Feng, X. M. Highly Enantioselective Conjugate Addition of Thioglycolate to Chalcones Catalyzed by Lanthanum: Low Catalyst Loading and Remarkable Chiral Amplification. Angew. Chem. Int. Ed. 2010, 49, 4290–4293.
- 29(a) Zheng, K.; Shi, J.; Liu, X. H.; Feng, X. M. Asymmetric Carbonyl-Ene Reaction Catalyzed by Chiral N,N'-Dioxide-Nickel(II) Complex: Remarkably Broad Substrate Scope. J. Am. Chem. Soc. 2008, 130, 15770–15771; (b) Zheng, K.; Liu, X. H.; Zhao, J. N.; Yang, Y.; Lin, L. L.; Feng, X. M. Highly Enantioselective Aza-Ene-Type Reaction Catalyzed by Chiral N,N'-Dioxide-Nicke(II) Complex. Chem. Commun. 2010, 46, 3771–3773; (c) Luo, W. W.; Zhao, J. N.; Yin, C. K.; Liu, X. H.; Lin, L. L.; Feng, X. M. Catalytic Hetero-Ene Reactions of 5-Methyleneoxazolines: Highly Enantioselective Synthesis of 2,5-Disubstituted Oxazole Derivatives. Chem. Commun. 2014, 50, 7524–7526.
- 30 Zheng, K.; Yin, C. K.; Liu, X. H.; Lin, L. L.; Feng, X. M. Catalytic Asymmetric Addition of Alkyl Enol Ethers to 1,2-Dicarbonyl Compounds: Highly Enantioselective Synthesis of Substituted 3-Alkyl-3-Hydroxyoxindoles. Angew. Chem. Int. Ed. 2011, 50, 2573–2577.
- 31(a) Zhou, L.; Liu, X. H.; Ji, J.; Zhang, Y. H.; Lin, L. L.; Feng, X. M. Enantioselective Baeyer-Villiger Oxidation: Desymmetrization of Meso Cyclic Ketones and Kinetic Resolution of Racemic 2-Arylcyclohexanones. J. Am. Chem. Soc. 2012, 134, 17023–17026; (b) Zhou, L.; Liu, X. H.; Ji, J.; Zhang, Y. H.; Wu, W. B.; Liu, Y. B.; Lin, L. L.; Feng, X. M. Regio- and Enantioselective Baeyer-Villiger Oxidation: Kinetic Resolution of Racemic 2-Substituted Cyclopentanones. Org. Lett. 2014, 16, 3938–3941.
- 32 Li, W.; Wang, J.; Hu, X. L.; Shen, K.; Wang, W. T.; Chu, Y. Y.; Lin, L. L.; Liu, X. H.; Feng, X. M. Catalytic Asymmetric Roskamp Reaction of α-Alkyl-α-diazoesters with Aromatic Aldehydes: Highly Enantioselective Synthesis of α-Alkyl-β-keto Esters. J. Am. Chem. Soc. 2010, 132, 8532–8533.
- 33 Cai, Y. F.; Liu, X. H.; Zhou, P. F.; Feng, X. M. Asymmetric Catalytic Halofunctionalization of α,β-Unsaturated Carbonyl Compounds. J. Org. Chem. 2019, 84, 1–13.
- 34(a) Xia, Y.; Lin, L. L.; Chang, F. Z.; Fu, X.; Liu, X. H.; Feng, X. M. Asymmetric Ring-Opening of Cyclopropyl Ketones with Thiol, Alcohol, and Carboxylic Acid Nucleophiles Catalyzed by a Chiral N,N'-Dioxide- Scandium(III) Complex. Angew. Chem. Int. Ed. 2015, 54, 13748–13752; (b) Xia, Y.; Chang, F. Z.; Lin, L. L.; Xu, Y. L.; Liu, X. H.; Feng, X. M. Asymmetric Ring-Opening of Cyclopropyl Ketones with β-Naphthols Catalyzed by a Chiral N,N'-Dioxide-Scandium(III) Complex. Org. Chem. Front. 2018, 5, 1293–1296; (c) Chang, F. Z.; Lin, L. L.; Xia, Y.; Zhang, H.; Dong, S. X.; Liu, X. H.; Feng, X. M. Chiral N,N'-Dioxide/ScIII-Complex- Catalyzed Asymmetric Ring-Opening Reaction of Cyclopropyl Ketones with Indoles. Adv. Synth. Catal. 2018, 360, 2608–2612; (d) Xia, Y.; Liu, X. H.; Zheng, H. F.; Lin, L. L.; Feng, X. M. Asymmetric Synthesis of 2,3-Dihydropyrroles by Ring-Opening/Cyclization of Cyclopropyl Ketones Using Primary Amines. Angew. Chem. Int. Ed. 2015, 54, 227–230; (e) Xia, Y.; Lin, L. L.; Chang, F. Z.; Liao, Y. T.; Liu, X. H.; Feng, X. M. Asymmetric Ring Opening/Cyclization/Retro-Mannich Reaction of Cyclopropyl Ketones with Aryl 1,2-Diamines for the Synthesis of Benzimidazole Derivatives. Angew. Chem. Int. Ed. 2016, 55, 12228–12232; (f) Fu, X.; Lin, L. L.; Xia, Y.; Zhou, P. F.; Liu, X. H.; Feng, X. M. Catalytic Asymmetric [3+3] Annulation of Cyclopropanes with Mercapto-acetaldehyde. Org. Biomol. Chem. 2016, 14, 5914–5917.
- 35 Holmquist, C. R.; Roskamp, E. J. A Selective Method for the Direct Conversion of Aldehydes into β-Keto Esters with Ethyl Diazoacetate Catalyzed by Tin(II) Chloride. J. Org. Chem. 1989, 54, 3258–3260.
- 36 Hassner, A.; Namboothiri, I. In Organic Syntheses Based on Name Reactions, Elsevier, Oxford, 2011, p. 408.
- 37(a) Li, W.; Liu, X. H.; Hao, X. Y.; Cai, Y. F.; Lin, L. L.; Feng, X. M. A Catalytic Asymmetric Ring-Expansion Reaction of Isatins and α-Alkyl-α-diazoesters: Highly Efficient Synthesis of Functionalized 2-Quinolone Derivatives. Angew. Chem. Int. Ed. 2012, 51, 8644–8647; (b) Li, W.; Tan, F.; Hao, X. Y.; Wang, G.; Tang, Y.; Liu, X. H.; Lin, L. L.; Feng, X. M. Catalytic Asymmetric Intramolecular Homologation of Ketones with α-Diazoesters: Synthesis of Cyclic α-Aryl/Alkyl β-Ketoesters. Angew. Chem. Int. Ed. 2015, 54, 1608–1611; (c) Li, W.; Liu, X. H.; Tan, F.; Hao, X. Y.; Zheng, J. F.; Lin, L. L.; Feng, X. M. Catalytic Asymmetric Homologation of α-Ketoesters with α-Diazoesters: Synthesis of Succinate Derivatives with Chiral Quaternary Centers. Angew. Chem. Int. Ed. 2012, 51, 8644–8647; (d) Li, W.; Liu, X. H.; Hao, X. Y.; Hu, X. L.; Chu, Y. Y.; Cao, W. D.; Qin, S.; Hu, C. W.; Lin, L. L.; Feng, X. M. New Electrophilic Addition of α-Diazoesters with Ketones for Enantio-selective C-N Bond Formation. J. Am. Chem. Soc. 2011, 133, 15268–15271.
- 38 Tan, F.; Liu, X. H.; Wang, Y.; Dong, S. X.; Yu, H.; Feng, X. M. Chiral Lewis Acid Catalyzed Reactions of α-Diazoester Derivatives: Construction of Dimeric Polycyclic Compounds. Angew. Chem. Int. Ed. 2018, 57, 16176–16179.
- 39(a) Lin, X. B.; Tang, Y.; Yang, W.; Tan, F.; Lin, L. L.; Liu, X. H.; Feng, X. M. Chiral Nickel(II) Complex Catalyzed Enantioselective Doyle-Kirmse Reaction of α-Diazo Pyrazoleamides. J. Am. Chem. Soc. 2018, 140, 3299–3305; (b) Lin, X. B.; Yang, W.; Yang, W. K.; Liu, X. H.; Feng, X. M. Asymmetric Catalytic [2,3] Stevens and Sommelet-Hauser Rearrangements of α-Diazo Pyrazoleamides with Sulfides. Angew. Chem. Int. Ed. 2019, 58, 13492–13498.
- 40(a) Cai, Y. F.; Liu, X. H.; Hui, Y. H.; Jiang, J.; Wang, W. T.; Chen, W. L.; Lin, L. L.; Feng, X. M. Catalytic Asymmetric Bromoamination of Chalcones: Highly Efficient Synthesis of Chiral α-Bromo-β-Amino Ketone Derivatives. Angew. Chem. Int. Ed. 2010, 49, 6160–6164; (b) Cai, Y. F.; Liu, X. H.; Li, J.; Chen, W. L.; Wang, W. T.; Lin, L. L.; Feng, X. M. Asymmetric Iodoamination of Chalcones and 4-Aryl-4-oxobutenoates Catalyzed by a Complex Based on Scandium(III) and a N,N'-Dioxide Ligand. Chem. Eur. J. 2011, 17, 14916–14921; (c) Cai, Y. F.; Liu, X. H.; Jiang, J.; Chen, W. L.; Lin, L. L.; Feng, X. M. Catalytic Asymmetric Chloroamination Reaction of α,β-Unsaturated γ-Keto Esters and Chalcones. J. Am. Chem. Soc. 2011, 133, 5636–5639; (d) Cai, Y. F.; Liu, X. H.; Zhou, P. F.; Kuang, Y. L.; Lin, L. L.; Feng, X. M. Iron-Catalyzed Asymmetric Haloamination Reactions. Chem. Commun. 2013, 49, 8054–8056; (e) Cai, Y. F.; Zhou, P. F.; Liu, X. H.; Zhao, J. N.; Lin, L. L.; Feng, X. M. Diastereoselectively Switchable Asymmetric Haloaminocyclization for the Synthesis of Cyclic Sulfamates. Chem. Eur. J. 2015, 21, 6386–6389; (f) Wang, Z. M.; Lin, L. L.; Zhou, P. F.; Liu, X. H.; Feng, X. M. Chiral N,N'-Dioxide-Sc(NTf2)3 Complex-Catalyzed Asymmetric Bromoamination of Chalones with N-Bromosuccinimide as Both Bromine and Amide Source. Chem. Commun. 2017, 53, 3462–3465.
- 41 Zhou, P. F.; Cai, Y. F.; Zhong, X.; Luo, W. W.; Kang, T. F.; Li, J.; Liu, X. H.; Lin, L. L.; Feng, X. M. Catalytic Asymmetric Intra- and Intermolecular Haloetherification of Enones: An Efficient Approach to (−)-Centrolobine. ACS Catal. 2016, 6, 7778–7783.
- 42 Li, W. W.; Zhou, P. F.; Li, G. L.; Lin, L. L.; Feng, X. M. Catalytic Asymmetric Halohydroxylation of α,β-Unsaturated Ketones with Water as the Nucleophile. Adv. Synth. Catal. 2020, 362, 1982–1987.
- 43(a) Zhou, P. F.; Lin, L. L.; Chen, L.; Zhong, X.; Liu, X. H.; Feng, X. M. Iron-Catalyzed Asymmetric Haloazidation of α,β-Unsaturated Ketones: Construction of Organic Azides with Two Vicinal Stereocenters. J. Am. Chem. Soc. 2017, 139, 13414–13419; (b) Zhou, P. F.; Liu, X. H.; Wu, W. B.; Xu, C. R.; Feng, X. M. Catalytic Asymmetric Construction of β-Azido Amides and Esters via Haloazidation. Org. Lett. 2019, 21, 1170–1175.
- 44(a) Zhao, X. H.; Liu, X. H.; Mei, H. J.; Guo, J.; Lin, L. L.; Feng, X. M. Asymmetric Dearomatization of Indoles through a Michael/Friedel- Crafts-Type Cascade to Construct Polycyclic Spiroindolines. Angew. Chem. Int. Ed. 2015, 54, 4032–4035; (b) Kang, T. F.; Hou, L. Z.; Ruan, S.; Cao, W. D.; Liu, X. H.; Feng, X. M. Lewis Acid-Catalyzed Asymmetric Reactions of β,γ-Unsaturated 2-Acyl Imidazoles. Nat. Commun. 2020, 11, 3869; (c) Zhang, D.; Su, Z. S.; He, Q. W.; Wu, Z. K.; Zhou, Y. Q.; Pan, C. J.; Liu, X. H.; Feng, X. M. Diversified Transformations of Tetrahydroindolizines to Construct Chiral 3-Arylindolizines and Dicarbofunctionalized 1,5-Diketones. J. Am. Chem. Soc. 2020, 142, 15975–15985.
- 45 Wang, P.-S.; Chen, D.-F.; Gong, L.-Z. Recent Progress in Asymmetric Relay Catalysis of Metal Complex with Chiral Phosphoric Acid. Top. Curr. Chem. 2020, 378, 9.
- 46 Li, J.; Lin, L. L.; Hu, B. W.; Lian, X. J.; Wang, G.; Liu, X. H.; Feng, X. M. Bimetallic Gold(I)/Chiral N,N'-Dioxide-Nickel(II) Asymmetric Relay Catalysis: Chemo- and Enantioselective Synthesis of Spiroketals and Spiroaminals. Angew. Chem. Int. Ed. 2016, 55, 6075–6078.
- 47 Li, J.; Lin, L. L.; Hu, B. W.; Zhou, P. F.; Huang, T. Y.; Liu, X. H.; Feng, X. M. Gold(I)/Chiral N,N'-Dioxide-Nickel(II) Relay Catalysis for Asymmetric Tandem Intermolecular Hydroalkoxylation/Claisen Rearrangement. Angew. Chem. Int. Ed. 2017, 56, 885–888.
- 48 Zheng, H. F.; Wang, Y.; Xu, C. R.; Xiong, Q.; Lin, L. L.; Feng, X. M. Diversified 1,6-Enynes Cycloisomerization/Diels-Alder Reactions via Bimetallic Relay Asymmetric Catalysis. Angew. Chem. Int. Ed. 2019, 58, 5327–5331.
- 49 Zhang, D.; Lin, L. L.; Yang, J.; Liu, X. H.; Feng, X. M. Asymmetric Synthesis of Tetrahydroindolizines by Bimetallic Relay Catalyzed Cycloaddition of Pyridinium Ylide. Angew. Chem. Int. Ed. 2018, 57, 12323–12327.
- 50 Yang, J.; Ke, C.; Zhang, D.; Liu, X. H.; Feng, X. M. Enantioselective Synthesis of 2,2,3-Trisubstituted Indolines via Bimetallic Relay Catalysis of α-Diazoketones with Enones. Org. Lett. 2018, 20, 4536–4539.
- 51 Zheng, H. F.; Wang, Y.; Xu, C.; Xiong, Q.; Lin, L. L.; Feng, X. M. Diversified Cycloisomerization/Diels-Alder Reactions of 1,6-Enynes through Bimetallic Relay Asymmetric Catalysis. Angew. Chem. Int. Ed. 2019, 58, 5327–5331.
- 52 Abbas, Z.; Dasaria, S.; Patra, A. K. Ternary Eu(III) and Tb(III) β-Diketonate Complexes Containing Chalcones: Photophysical Studies and Biological Outlook. RSC Adv. 2017, 7, 44272–44281.
- 53 Yu, H.; Dong, S. X.; Yao, Q.; Chen, L.; Zhang, D.; Liu, X. H.; Feng, X. M. Enantioselective [2+2] Photocycloaddition Reactions of Enonesand Olefins with Visible Light Mediated by N,N'-Dioxide-Metal Complexes. Chem. Eur. J. 2018, 24, 19361–19367.
- 54 Zhang, X. Y.; Wu, W. B.; Cao, W. D.; Yu, H.; Xu, X.; Liu, X. H.; Feng, X. M. Enantioselective Radical-Polar Crossover Reactions of Indanonecarboxamides with Alkenes. Angew. Chem. Int. Ed. 2020, 59, 4846–4850.
- 55(a) Kokubo, M.; Ogawa, C.; Kobayashi, S. Lewis Acid Catalysis in Water with a Hydrophilic Substrate: Scandium-Catalyzed Hydroxymethylation with Aqueous Formaldehyde in Water. Angew. Chem. Int. Ed. 2008, 47, 6909–6911; (b) Kobayashi, S.; Kokubo, M.; Kawasumi, K.; Nagano, T. Chiral Scandium-Catalyzed Enantioselective Hydroxymethylation of Ketones in Water. Chem. Asian J. 2010, 5, 490–492.
- 56 Boomhoff, M.; Schneider, C. A Novel Three-Component [3+2] Cycloannulation Process for the Rapid and Highly Stereoselective Synthesis of Pyrrolobenzoxazoles. Chem. Eur. J. 2012, 18, 4185–4189.
- 57(a) Maji, B.; Baidya, M.; Yamamoto, H. Asymmetric Construction of Quaternary Stereocenters by Magnesium Catalysed Direct Amination of β-Ketoesters using in situ Generated Nitrosocarbonyl Compounds as Nitrogen Sources. Chem. Sci. 2014, 5, 3941–3945; (b) Baidya, M.; Griffin, K. A.; Yamamoto, H. Catalytic Enantioselective O-Nitrosocarbonyl Aldol Reaction of β-Dicarbonyl Compounds. J. Am. Chem. Soc. 2012, 134, 18566–18569.
- 58 Wang, C.; Yamamoto, H. Gadolinium-Catalyzed Regio- and Enantioselective Aminolysis of Aromatic trans-2,3-Epoxy Sulfonamides. Angew. Chem. Int. Ed. 2015, 54, 8760–8763.
- 59 Yao, L.; Zhu, Q.; Wei, L.; Wang, Z.-F.; Wang, C.-J. Dysprosium(III)-Catalyzed Ring-Opening of meso-Epoxides: Desymmetrization by Remote Stereocontrol in a Thiolysis/Elimination Sequence. Angew. Chem. Int. Ed. 2016, 55, 5829–5833.
- 60 Li, L.; Yang, Q.; Wang, Y.; Jia, Y. Catalytic Asymmetric Total Synthesis of (-)-Galanthamine and (-)-Lycoramine. Angew. Chem. Int. Ed. 2015, 54, 6255–6259.
- 61 Jayakumar, S.; Louven, K.; Strohmann, C.; Kumar, K. A Tunable and Enantioselective Hetero-Diels-Alder Reaction Provides Access to Distinct Piperidinoyl Spirooxindoles. Angew. Chem. Int. Ed. 2017, 56, 15945–15949.
- 62 He, W.; Hu, J.; Wang, P.; Chen, L.; Ji, K.; Yang, S.; Li, Y.; Xie, Z.; Xie, W. Highly Enantioselective Tandem Michael Addition of Tryptamine-Derived Oxindoles to Alkynones: Concise Synthesis of Strychnos Alkaloids. Angew. Chem. Int. Ed. 2018, 57, 3806–3809.
- 63 Ye, C.-X.; Melcamu, Y. Y.; Li, H.-H.; Cheng, J.-T.; Zhang, T.-T.; Ruan, Y.-P.; Zheng, X.; Lu, X.; Huang, P.-Q. Dual Catalysis for Enantioselective Convergent Synthesis of Enantiopure Vicinal Amino Alcohols. Nat. Commun. 2018, 9, 410.
- 64 Jia, Z.-J.; Shan, G.; Daniliuc, C. G.; Antonchick, A. P.; Waldmann, H. Enantioselective Synthesis of the Spirotropanyl Oxindole Scaffold through Bimetallic Relay Catalysis. Angew. Chem. Int. Ed. 2018, 57, 14493–14497.
- 65 Yang, H.; Xi, C.; Miao, Z.; Chen, R. Cross-Coupling Reactions of Aryl Halides with Amines, Phenols, and Thiols Catalyzed by an N,N'-Dioxide-Copper(I) Catalytic System. Eur. J. Org. Chem. 2011, 3353–3360.
- 66(a) Chakka, S. K.; Cele, Z. E. D.; Sosibo, S. C.; Francis, V.; Arvidsson, P. I.; Kruger, H. G.; Maguire, G. E. M.; Govender, T. Asymmetric Conjugate Addition of Thioglycolate to a Range of Chalcones Using Tetrahydroisoquinoline (TIQ) N,N'-Dioxide Ligands. Tetrahedron: Asymmetry 2012, 23, 616–622; (b) Cele, Z. E. D.; Sosibo, S. C.; Andersson, P. G.; Kruger, H. G.; Maguire, G. E. M.; Govender, T. Catalytic Asymmetric Carbon-Carbon Bond Forming Reactions Catalyzed by Tetrahydroisoquinoline (TIQ) N,N'-Dioxide Ligands. Tetrahedron: Asymmetry 2013, 24, 191–195.
- 67 Kitanosono, T.; Kobayashi, S. Toward Chemistry-Based Design of the Simplest Metalloenzyme-Like Catalyst That Works Efficiently in Water. Chem. Asian J. 2015, 10, 133–138.
- 68 Gao, E.; Li, M.; Duan, L.; Li, L.; Li, Y.-M. Isosteric Expansion of the Structural Diversity of Chiral Ligands: Design and Application of Proline-Based N,N'-Dioxide Ligands for Copper-Catalyzed Enantioselective Henry Reactions. Tetrahedron 2019, 75, 130492.
- 69 Qin, B.; Xiao, X.; Liu, X. H.; Huang, J. L.; Wen, Y. H.; Feng, X. M. Highly Enantioselective Henry (Nitroaldol) Reaction of Aldehydes and α-Ketoesters Catalyzed by N,N'-Dioxide-Copper(I) Complexes. J. Org. Chem. 2007, 72, 9323–9328.




