Chen's Reagent: A Versatile Reagent for Trifluoromethylation, Difluoromethylenation, and Difluoroalkylation in Organic Synthesis†
Qiqiang Xie
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Jinbo Hu
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorQiqiang Xie
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Jinbo Hu
Key Laboratory of Organofluorine Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling-Ling Road, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorSummary
Methyl fluorosulfonyldifluoroacetate (FSO2CF2CO2Me or MFSDA), often called “Chen's reagent”, is commonly used to synthesize trifluoromethylated and difluoroalkylated compounds. This important reagent was initially developed as an efficient trifluoromethylating agent by Professor Qing-Yun Chen and co-workers at Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences in 1989. Since then, this reagent has been widely used in academia and industry for the copper-mediated trifluoromethylation of aryl, alkenyl, and even some alkyl halides, among others. During the last decade, this reagent was further developed as a difluorocarbene precursor as well as a radical difluoroalkylating agent under visible light promoted redox catalysis. This review aims to briefly highlight the initial discovery, historical development, and synthetic applications of Chen's reagent, and provide some guidelines for readers to use Chen's reagent in their own synthesis.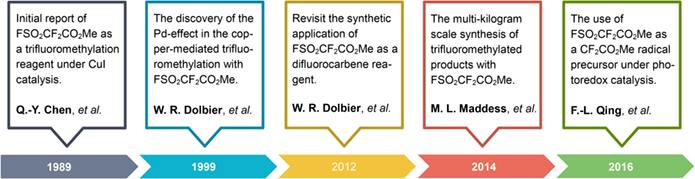
References
- 1 O'Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319.
- 2
Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed., WILEY-VCH, Weinheim, 2013.
10.1002/9783527651351 Google Scholar
- 3(a) Wang, J.; Sanchez-Rosello, M.; Acena, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001-2011). Chem. Rev. 2014, 114, 2432–2506; (b) Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359; (c) Mei, H.; Han, J.; Fustero, S.; Medio-Simon, M.; Sedgwick, D. M.; Santi, C.; Ruzziconi, R.; Soloshonok, V. A. Fluorine-Containing Drugs Approved by the FDA in 2018. Chem. Eur. J. 2019, 25, 11797–11819.
- 4(a) Jeschke, P. The unique role of fluorine in the design of active ingredients for modern crop protection. ChemBioChem 2004, 5, 570–589; (b) Fujiwara, T.; O'Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluorine Chem. 2014, 167, 16–29.
- 5 Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508.
- 6(a) McClinton, M. A.; McClintion, D. A. Trifluoromethylation and Related Reactions in Organic Chemistry. Tetrahedron 1992, 48, 6555–6666; (b) Schlosser, M. CF3-bearing aromatic and heterocyclic building blocks. Angew. Chem. Int. Ed. 2006, 45, 5432–5446; (c) Furuya, T.; Kamlet, A. S.; Ritter, T. Catalysis for fluorination and trifluoromethylation. Nature 2011, 473, 470–477; (d) Qing, F.-L. Recent Advances of Trifluoromethylation. Chin. J. Org. Chem. 2012, 32, 815–824; (e) Studer, A. A "Renaissance" in radical trifluoromethylation. Angew. Chem. Int. Ed. 2012, 51, 8950–8958; (f) Zhang, C. Recent advances in trifluoromethylation of organic compounds using Umemoto's reagents. Org. Biomol. Chem. 2014, 12, 6580–6589; (g) Ma, J.-A.; Cahard, D. Strategies for nucleophilic, electrophilic, and radical trifluoromethylations. J. Fluorine Chem. 2007, 128, 975–996; (h) Tomashenko, O. A.; Grushin, V. V. Aromatic trifluoromethylation with metal complexes. Chem. Rev. 2011, 111, 4475–4521; (i) Liu, X.; Xu, C.; Wang, M.; Liu, Q. Trifluoromethyltrimethylsilane: nucleophilic trifluoromethylation and beyond. Chem. Rev. 2015, 115, 683–730; (j) Ni, C.; Hu, M.; Hu, J. Good partnership between sulfur and fluorine: sulfur-based fluorination and fluoroalkylation reagents for organic synthesis. Chem. Rev. 2015, 115, 765–825; (k) Oishi, M.; Kondo, H.; Amii, H. Aromatic trifluoromethylation catalytic in copper. Chem. Commun. 2009, 1909–1911; (l) Rao, Z.-P.; Sun, Y.-Y.; Zhou, X.-F.; Xie, Q.; Zhu, H.-X.; Dai, J.-J.; Xu, J.; Xu, H.-J. Efficient AcrH2 catalyzed β-trifluoromethylation of carbonyl compounds by atom transfer radical addition reactions. Chin. J. Chem. 2019, 37, 1025–1030; (m) Su, Z.; Guo, Y.; Chen, Q.-Y.; Zhao, Z.-G.; Nian, B.-Y. Catalyst-free hydrotrifluoromethylation of alkenes using iodotrifluoromethane. Chin. J. Chem. 2019, 37, 597–604; (n) Wang, Q.; Gao, K.; Zou, J.; Zeng, R. Copper(I)-catalyzed non-terminal enamides trifluoromethylation: flexible synthesis of N-(3,3,3-trifluoro-2-arylprop-1-en-1-yl) substituted benzamide. Chin. J. Org. Chem. 2018, 38, 863–870; (o) Chen, D.; Yang, W.; Yao, Y.; Yang, X.; Deng, Y.; Yang, D. Recent advances in transition metal-promoted trifluoromethylation reactions. Chin. J. Org. Chem. 2018, 38, 2571–2589; (p) Ji, X.; Shi, G.; Zhang, Y. Progress of trifluoromethylation using trifluoroacetic acid and its derivatives as CF3-sources. Chin. J. Org. Chem. 2019, 39, 929–939.
- 7
Chen, Q.-Y.; Wu, S.-W. Methyl fluorosulphonyldifluoroacetate; a new trifluoromethylating agent. J. Chem. Soc., Chem. Commun. 1989, 705–706.
10.1039/c39890000705 Google Scholar
- 8(a) Chen, Q.-Y. Trifluoromethylation of organic halides with difluorocarbene precursors. J. Fluorine Chem. 1995, 72, 241–246; (b) Chen, Q.-Y. Some Progress in Organofluorine Chemistry Promoted by the Preparation of Chromic Acid Mist Suppressant, F-53. Chin. J. Org. Chem. 2001, 21, 805–809; (c) Zhang, C.-P.; Chen, Q.-Y.; Guo, Y.; Xiao, J.-C.; Gu, Y.-C. Difluoromethylation and trifluoromethylation reagents derived from tetrafluoroethane β-sultone: Synthesis, reactivity and applications. Coord. Chem. Rev. 2014, 261, 28–72.
- 9 Clarke, S. L.; McGlacken, G. P. Methyl fluorosulfonyldifluoroacetate (MFSDA): An Underutilised Reagent for Trifluoromethylation. Chem. Eur. J. 2017, 23, 1219–1230.
- 10 Olah, G. A.; Iyer, P. S.; Prakash, G. K. S. Perfluorinated Resinsulfonic Acid (Nafion-H®) Catalysis in Synthesis. Synthesis 1986, 513–531.
- 11 Chiang, S. H.-K.; Davis, H. R. Fluorine-Containing Carbyl Sulfates and Their Production. US Patent: original application on August 5, 1955, Ser. No. 526769; divided and application on May 17, 1963, Ser. No. 302221; Patented on October 26, 1965, US 3214443.
- 12 Anderson, J. L.; England, D. C. Fluorosultones and Sulfites, and the Preparation of the Latter. US Patent: filed on December 1955, Ser. No. 552224; Patented on December 29, 1964, US 3163656.
- 13 Jiang, S. H.-K. The addition reactions of sulfur trioxide to carbon- carbon double bonds I. Acta Chim. Sinica 1957, 23, 330–339.
- 14(a) England, D. C. α–Sulfopolyfluoromonocarboxylic acids and derivatives hydrolyzable thereto. US 2852554, 1958; (b) England, D. C.; Dietrich, M. A.; Lindsey, R. V. Reactions of Fluoroolefins with Sulfur Trioxide. J. Am. Chem. Soc. 1960, 82, 6181–6188.
- 15(a) Dmitriev, M. A.; Sokol'skii, G. A.; Knunyants, I. L. Fluorine-containing β-sultones Communication 1. Addition of sulfur trioxide to fluoro olefins. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1960, 9, 792–795;
10.1007/BF01179175 Google Scholar(b) Dmitriev, M. A.; Sokol'skii, G. A.; Knunyants, I. L. Fluorine-containing β-sultones Communication 2. Hydrolysis of tetrafluoro-2-hydroxyethanesulfonic acid β-sultone. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1960, 9, 966–969;10.1007/BF00903970 Google Scholar(c) Zhao, G.; Wu, H.; Xiao, Z.; Chen, Q.-Y.; Liu, C. Trifluoromethylation of haloarenes with a new trifluoro-methylating reagent Cu(O2CCF2SO2F)2. RSC Adv. 2016, 6, 50250–50254.
- 16 Huang, W.-Y.; Chen, Q.-Y. Perfluoroalkanesulfonic acids and their derivatives. In The Chemistry of Sulphonic Acids, Esters and Their Derivatives, Eds.: Patai, S.; Rappoport, Z., John Wiley & Sons, N. Y., 1991, p. 903.
- 17 Chen, Q.-Y.; Zhu, S.-Z. Perfluoro- and Polyfluorosulfonic Acids (XV)—Generation of Difluorocarbene and Fluorosulfonyldifluoromethide Ion From Methyl α-Fluorosulfonyldifluoroacetate. Scientia Sinica B (Chinese version) 1986, 561–568; Scientia Sinica B 1987, 30, 561–571.
- 18 Wu, S.-W. Difluorocarbene Chemistry: Research of New difluorocarbene Precursors and Their Reactivity. Ph.D. Dissertation, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 1989 (in Chinese).
- 19 Chen, Q.-Y.; Yang, G.-Y.; Wu, S.-W. Copper electron-transfer induced trifluoromethylation with methyl fluorosulphonyldifluoroacetate. J. Fluorine Chem. 1991, 55, 291–298.
- 20 Wiemers, D. M.; Burton, D. J. Pregeneration, Spectroscopic Detection, and Chemical Reactivity of (Trifluoromethyl)copper, an Elusive and Complex Species. J. Am. Chem. Soc. 1986, 108, 832.
- 21 Matsui, J. K.; Lang, S. B.; Heitz, D. R.; Molander, G. A. Photoredox- Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development. ACS Catal. 2017, 7, 2563–2575.
- 22 Yu, W.; Xu, X.-H.; Qing, F.-L. Photoredox Catalysis Mediated Application of Methyl Fluorosulfonyldifluoroacetate as the CF2CO2R Radical Source. Org. Lett. 2016, 18, 5130–5133.
- 23 Chen, Q.-Y.; Duan, J.-X. Direct trifluoromethylthiolation of aryl halides using methyl fluorosulfonyldifluoroacetate and sulfur. J. Chem. Soc., Chem. Commun. 1993, 918–919.
- 24
Qing, F.-L.; Fan, J.; Sun, H.-B.; Yue, X.-J. First synthesis of ortho-trifluoromethylated aryl triflates. J. Chem. Soc., Perkin Trans. 1, 1997, 3053–3058.
10.1039/a702607b Google Scholar
- 25(a) Zhang, X.; Qing, F.-L.; Yang, Y.; Yu, J.; Fu, X.-K. A new route to α-trifluoromethyl-α,β-unsaturated esters. Tetrahedron Lett. 2000, 41, 2953–2955; (b) Zhang, X.; Qing, F.-L.; Yu, Y. Synthesis of 2‘,3‘-Dideoxy-2‘-trifluoromethylnucleosides from α-Trifluoromethyl- α,β-unsaturated Ester. J. Org. Chem. 2000, 65, 7075–7082.
- 26(a) Fei, X.-S.; Tian, W.-S.; Chen, Q.-Y. Synthesis of 4-trifluoromethylsteroids: A novel class of steroid 5α-reductase inhibitors. Bioorg. Med. Chem. Lett. 1997, 7, 3113–3118; (b) Fei, X.-S.; Tian, W.-S.; Chen, Q.-Y. New, convenient route for trifluoromethylation of steroidal molecules. J. Chem. Soc., Perkin Trans. 1 1998, 1139–1142.
- 27 Roche, A. J.; Dolbier, W. R. Electrophilic Substitution of 1,1,2,2,9,9, 10,10-Octafluoro[2.2]paracyclophane. J. Org. Chem. 1999, 64, 9137–9143.
- 28 Roche, A. J.; Dolbier, W. R. Multiple Electrophilic Substitution of 1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophane. J. Org. Chem. 2000, 65, 5282–5290.
- 29 Qing, F.-L.; Zhang, X.; Peng, Y. The trifluoromethylation of 1,1-dibromo-1-alkenes using trifluoromethylcopper (CF3Cu) generated in situ from methyl fluorosulfonyldifluoroacetate. J. Fluorine Chem. 2001, 111, 185–187.
- 30 Liu, C.; Chen, Q.-Y. Fluoroalkylation of Porphyrins: A Facile Synthesis of Trifluoromethylated Porphyrins by a Palladium-Catalyzed Cross- Coupling Reaction. Eur. J. Org. Chem. 2005, 2005, 3680–3686.
- 31 Véliz, E. A.; Stephens, O. M.; Beal, P. A. Synthesis and Analysis of RNA Containing 6-Trifluoromethylpurine Ribonucleoside. Org. Lett. 2001, 3, 2969–2972.
- 32 Kobayashi, Y.; Yamamoto, K.; Asai, T.; Nakano, M.; Kumadaki, I. Studies on organic fluorine compounds. Part 35. Trifluoromethylation of pyrimidine- and purine-nucleosides with trifluoromethyl–copper complex. J. Chem. Soc., Perkin Trans. 1 1980, 2755–2761.
- 33 Romero, F. A.; Hwang, I.; Boger, D. L. Delineation of a Fundamental α-Ketoheterocycle Substituent Effect for Use in the Design of Enzyme Inhibitors. J. Am. Chem. Soc. 2006, 128, 14004–14005.
- 34(a) Foster, R. S.; Jakobi, H.; Harrity, J. P. A. A General and Regioselective Synthesis of 5-Trifluoromethyl-pyrazoles. Org. Lett. 2012, 14, 4858–4861; (b) Foster, R. S.; Adams, H.; Jakobi, H.; Harrity, J. P. A. Synthesis of 4-Fluoromethylsydnones and their Participation in Alkyne Cycloaddition Reactions. J. Org. Chem. 2013, 78, 4049–4064.
- 35 Chong, P.; Davis, R.; Elitzin, V.; Hatcher, M.; Liu, B.; Salmons, M.; Tabet, E. Synthesis of 7-trifluoromethylpyrazolo[1,5-a]pyridinedicarboxylate. Tetrahedron Lett. 2012, 53, 6786–6788.
- 36 Maddess, M. L.; Scott, J. P.; Alorati, A.; Baxter, C.; Bremeyer, N.; Brewer, S.; Campos, K.; Cleator, E.; Dieguez-Vazquez, A.; Gibb, A.; Gibson, A.; Howard, M.; Keen, S.; Klapars, A.; Lee, J.; Li, J.; Lynch, J.; Mullens, P.; Wallace, D.; Wilson, R. Enantioselective Synthesis of a Highly Substituted Tetrahydrofluorene Derivative as a Potent and Selective Estrogen Receptor Beta Agonist. Org. Proc. Res. Dev. 2014, 18, 528–538.
- 37 Schnute, M. E.; Wennerstål, M.; Alley, J.; Bengtsson, M.; Blinn, J. R.; Bolten, C. W.; Braden, T.; Bonn, T.; Carlsson, B.; Caspers, N.; Chen, M.; Choi, C.; Collis, L. P.; Crouse, K.; Färnegårdh, M.; Fennell, K. F.; Fish, S.; Flick, A. C.; Goos-Nilsson, A.; Gullberg, H.; Harris, P. K.; Heasley, S. E.; Hegen, M.; Hromockyj, A. E.; Hu, X.; Husman, B.; Janosik, T.; Jones, P.; Kaila, N.; Kallin, E.; Kauppi, B.; Kiefer, J. R.; Knafels, J.; Koehler, K.; Kruger, L.; Kurumbail, R. G.; Kyne, R. E.; Li, W.; Löfstedt, J.; Long, S. A.; Menard, C. A.; Mente, S.; Messing, D.; Meyers, M. J.; Napierata, L.; Nöteberg, D.; Nuhant, P.; Pelc, M. J.; Prinsen, M. J.; Rhönnstad, P.; Backström-Rydin, E.; Sandberg, J.; Sandström, M.; Shah, F.; Sjöberg, M.; Sundell, A.; Taylor, A. P.; Thorarensen, A.; Trujillo, J. I.; Trzupek, J. D.; Unwalla, R.; Vajdos, F. F.; Weinberg, R. A.; Wood, D. C.; Xing, L.; Zamaratski, E.; Zapf, C. W.; Zhao, Y.; Wilhelmsson, A.; Berstein, G. Discovery of 3-Cyano-N-(3-(1-isobutyrylpiperidin-4-yl)-1-methyl-4- (trifluoromethyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)benzamide: A Potent, Selective, and Orally Bioavailable Retinoic Acid Receptor-Related Orphan Receptor C2 Inverse Agonist. J. Med. Chem. 2018, 61, 10415–10439.
- 38 Junges, A. F.; Pittaluga, E. P.; Zanatta, N.; Martins, M. A. P.; Bonacorso, H. G. Novel 4,5-bis(trifluoromethyl)-1H-pyrazoles through a concise sequential iodination-trifluoromethylation reaction. Tetrahedron Lett. 2019, 60, 1385–1388.
- 39(a) Zhao, S.; Guo, Y.; Han, E.-J.; Luo, J.; Liu, H.-M.; Liu, C.; Xie, W.; Zhang, W.; Wang, M. Copper(II)-catalyzed trifluoromethylation of iodoarenes using Chen's reagent. Org. Chem. Front. 2018, 5, 1143–1147; and references therein; (b) Mu, Y.; Wan, X. A facile and efficient synthesis of new fluoroalkylsulfonates and the corresponding tetrabutylammonium salts. Tetrahedron Lett. 2019, 60, doi.org/10.1016/j. tetlet.2019.150966; (c) Sudhakar, K.; Mahammed, A.; Fridman, N.; Gross, Z. Trifluoromethylation for affecting the structural, electronic and redox properties of cobalt corroles. Dalton Trans. 2019, 48, 4798–4810.
- 40 Cao, P.; Duan, J.-X.; Chen, Q.-Y. Difluoroiodomethane: practical synthesis and reaction with alkenes. J. Chem. Soc., Chem. Commun. 1994, 737–738.
- 41 Eusterwiemann, S.; Martinez, H.; Dolbier, W. R. Methyl 2,2-Difluoro- 2-(fluorosulfonyl)acetate, a Difluorocarbene Reagent with Reactivity Comparable to That of Trimethylsilyl 2,2-Difluoro-2-(fluorosulfonyl)acetate (TFDA). J. Org. Chem. 2012, 77, 5461–5464.
- 42 Thomoson, C. S.; Martinez, H.; Dolbier, W. R. The use of methyl 2,2-difluoro-2-(fluorosulfonyl)acetate as the difluorocarbene source to generate an in situ source of difluoromethylene triphenylphosphonium ylide. J. Fluorine Chem. 2013, 150, 53–59.
- 43 Yu, W.; Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Visible Light-Induced Methoxycarbonyldifluoromethylation of Trimethylsilyl Enol Ethers and Allyltrimethylsilanes with FSO2CF2CO2Me. Chin. J. Chem. 2018, 36, 1024–1030.
- 44 Luo, X.; Fan, Z.; Zhang, B.; Chen, C.; Xi, C. Visible-light-triggered direct keto-difluoroacetylation of styrenes with (fluorosulfonyl)difluoroacetate and dimethyl sulfoxide leads to α-difluoroacetylated ketones. Chem. Commun. 2019, 55, 10980–10983.
- 45(a) Chen, Q.-Y.; Wu, S.-W. A simple convenient method for preparation of difluoromethyl ethers using fluorosulfonyldifluoroacetic acid as a difluorocarbene precursor. J. Fluorine Chem. 1989, 44, 433–440; (b) Chen, Q.-Y.; Wu, S.-W. Perfluoro- and polyfluorosulfonic acids. 21. Synthesis of difluoromethyl esters using fluorosulfonyldifluoroacetic acid as a difluorocarbene precursor. J. Org. Chem. 1989, 54, 3023–3027; (c) Chen, Q.-Y.; Yang, G.-Y.; Wu, S.-W. Some reactions of fluorosulfonyldifluoroacetic acid with N-heterocyclic compounds. Chin. J. Chem . 1992, 10, 350–354.
- 46(a) Su, D.-B.; Zhu, R.-X.; Qiu, Z.-M.; Chen, Q.-Y. Studies on Fluoroalkylation and Fluoroalkoxylation 32. The Addition Reaction of Difluoroiodomethane Sulfonyl Fluoride with Olefin in the Presence of Copper--Coexistence of Radical and Carbene Process Induced by Electron Transfer. Acta Chim. Sinica 1990, 48, 596–601; (b) Chen, Q.-Y.; Wu, S.-W. Studies on fluoroalkylation and fluoroalkoxylation. Part 33. Direct trifluoromethylation of aryl halides with fluorosulphonyldifluoromethyl iodide in the presence of copper: an electron transfer induced process. J. Chem. Soc., Perkin Trans. 1, 1989, 2385–2387.
- 47(a) Xiao, J.-C.; Duan, J.-X.; Li, A.-R.; Guo, Y.; Chen, Q.-Y. A simple method for preparing difluorodiiodomethane from difluoro(fluorosulfonyl)acetyl fluoride. Collect. Czech. Chem. Commun. 2002, 67, 1320–1324; (b) Chen, Q.-Y.; Li, Z.-T. Photoinduced electron-transfer reaction of difluorodiiodomethane with aza-aromatic compounds and enamines. J. Chem. Soc. , Perkin Trans. I 1993, 645–648; (c) Li, A.-R.; Chen, Q.-Y. Lead tetraacetate induced addition reaction of difluorodiiodomethane to alkenes and alkynes. Synthesis of fluorinated telechelic compounds. Synthesis 1997, 1481–1488.
- 48(a) Chen, Q.-Y.; Duan, J.-X. Methyl 3-oxo-ω-fluorosulfonylperfluoropentanoate: a versatile trifluoromethylating agent for organic halides. J. Chem. Soc., Chem. Commun. 1993, 1389–1391;
10.1039/C39930001389 Google Scholar(b) Long, Z.-Y.; Duan, J.-X.; Lin, Y.-B.; Guo, C.-Y.; Chen, Q.-Y. Potassium 3-oxa-ω-fluorosulfonylperfluoropentanoate (FO2SCF2CF2OCF2CO2K), a low-temperature trifluoromethylating agent for organic halides; its α-carbon- oxygen bond fragmentation. J. Fluorine Chem. 1996, 78, 177–181.
- 49
Duan, J.-X.; Chen, Q.-Y. Copper induced single electron transfer trifluoromethylation of organic halides with 3-oxo-ω-fluorosulfonylperfluoropentyl iodide. Chin. J. Chem. 1994, 12, 464–467.
10.1002/cjoc.19940120511 Google Scholar
- 50(a) Tian, F.; Kruger, V.; Bautista, O.; Duan, J.-X.; Li, A.-R.; Dolbier, W. R.; Chen, Q.-Y. A Novel and Highly Efficient Synthesis of gem-Difluorocyclopropanes. Org. Lett. 2000, 2, 563–564;
(b) Dolbier Jr, W. R.; Tian, F.; Duan, J.-X.; Li, A.-R.; Ait-Mohand, S.; Bautista, O.; Buathong, S.; Marshall Baker, J.; Crawford, J.; Anselme, P.; Cai, X. H.; Modzelewska, A.; Koroniak, H.; Battiste, M. A.; Chen, Q.-Y. Trimethylsilyl fluorosulfonyldifluoroacetate (TFDA): a new, highly efficient difluorocarbene reagent. J. Fluorine Chem. 2004, 125, 459–469;
(c) Chen, Q.-Y. Trimethylsilyl Fluorosulfonyldifluoroacetate. In Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd., 2005.
10.1002/047084289X.rn00530 Google Scholar
- 51 Liu, Y.; Wu, H.; Guo, Y.; Xiao, J.-C.; Chen, Q.-Y.; Liu, C. Trifluoromethylfluorosulfonylation of Unactivated Alkenes Using Readily Available Ag(O2CCF2SO2F) and N-Fluorobenzenesulfonimide. Angew. Chem. Int. Ed. 2017, 56, 15432–15435.
- 52(a) Su, D.-B.; Duan, J.-X.; Chen, Q.-Y. Methyl chlorodifluoroacetate a convenient trifluoromethylating agent. Tetrahedron Lett. 1991, 32, 7689–7690;
(b) Duan, J.-X.; Su, D.-B.; Chen, Q.-Y. Trifluoromethylation of organic halides with methyl halodifluoroacetates – a process via difluorocarbene and trifluoromethide intermediates. J. Fluorine Chem. 1993, 11, 279–284;
10.1016/S0022-1139(00)80112-8 Google Scholar(c) Li, H.-D.; Duan, J.-X.; Su, D.-B.; Chen, Q.-Y. Study on potassium bromodifluoroacetate convenient approaches to the syntheses of trifluoromethylated compounds. Chin. J. Chem. 1993, 11, 366–369.
- 53 Levchenko, K.; Datsenko, O. P.; Serhiichuk, O.; Tolmachev, A.; Iaroshenko, V. O.; Mykhailiuk, P. K. Copper-Catalyzed O-Difluoromethylation of Functionalized Aliphatic Alcohols: Access to Complex Organic Molecules with an OCF2H Group. J. Org. Chem. 2016, 81, 5803–5813.
- 54(a) Xie, Q.; Ni, C.; Zhang, R.; Li, L.; Rong, J.; Hu, J. Efficient Difluoromethylation of Alcohols Using TMSCF2Br as a Unique and Practical Difluorocarbene Reagent under Mild Conditions. Angew. Chem. Int. Ed. 2017, 56, 206–3210; (b) Liu, G.-K.; Li, X.; Qin, W.-B.; Peng, X.-S.; Wong, H. N. C.; Zhang, L.; Zhang, X. Facile difluoromethylation of aliphatic alcohols with an S-(difluoro-methyl)sulfonium salt: reaction, scope and mechanistic study. Chem. Commun. 2019, 55, 7446–7449; (c) Zhu, J.; Liu, Y.; Shen, Q. Direct Difluoromethylation of Alcohols with an Electrophilic Difluoromethylated Sulfonium Ylide. Angew. Chem. Int. Ed. 2016, 55, 9050–9054.
- 55The prices of Chen's reagent and TMSCF3 were obtained in November 2019 by searching in the following website: https://www.sigmaaldrich.com/united-states.html.
References
- 1(a) Chen, Q.-Y. Scientia Sinica 1978, 21, 773; (b) Chen, Q.-Y. Chin. J. Org. Chem. 2001, 21, 805.
- 2(a) Chen, Q.-Y.; Wu, S.-W. J. Chem. Soc., Chem. Commun. 1989, 705; (b) Chen, Q.-Y. J. Fluorine Chem. 1995, 72, 241; (c) Zhang, C.-P.; Chen, Q.-Y.; Guo, Y.; Xiao, J.-C.; Gu, Y.-C. Coord. Chem. Rev. 2014, 261, 28; (d) Liu, Y.; Wu, H.; Guo, Y.; Xiao, J.-C.; Chen, Q.-Y.; Liu, C. Angew. Chem. Int. Ed. 2017, 56, 15432.
- 3(a) Chen, Q.-Y. Israel J. Chem. 1999, 39, 179; (b) Zhang, C.-P.; Chen, Q.-Y.; Guo, Y.; Xiao, J.-C.; Gu, Y.-C. Chem. Soc. Rev. 2012, 41, 4536; (c) Tang, X.-J.; Chen, Q.-Y. Chem. Sci. 2012, 3, 1694.
- 4(a) Jin, L.-M.; Zeng, Z.; Guo, C.-C.; Chen, Q.-Y. J. Org. Chem. 2003, 68, 3912; (b) Zeng, Z.; Liu, C.; Jin, L.-M.; Guo, C.-C.; Chen, Q.-Y. Eur. J. Org. Chem. 2005, 306; (c) Liu, C.; Shen, D.-M.; Chen, Q.-Y. J. Am. Chem. Soc. 2007, 129, 5814.




