Visible Light Accelerated Vinyl C–H Arylation in Pd-Catalysis: Application in the Synthesis of ortho Tetra-substituted Vinylarene Atropisomers
Jia Feng
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
These authors contributed equally to this work.Search for more papers by this authorBin Li
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
These authors contributed equally to this work.Search for more papers by this authorJulong Jiang
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
Search for more papers by this authorMingkai Zhang
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
Search for more papers by this authorWenbai Ouyang
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
Search for more papers by this authorChunyu Li
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
Search for more papers by this authorCorresponding Author
Yao Fu
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zhenhua Gu
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
E-mail: [email protected]; [email protected]Search for more papers by this authorJia Feng
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
These authors contributed equally to this work.Search for more papers by this authorBin Li
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
These authors contributed equally to this work.Search for more papers by this authorJulong Jiang
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
Search for more papers by this authorMingkai Zhang
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
Search for more papers by this authorWenbai Ouyang
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
Search for more papers by this authorChunyu Li
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
Search for more papers by this authorCorresponding Author
Yao Fu
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zhenhua Gu
Hefei National Laboratory for Physical Sciences at the Microscale, Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, China
E-mail: [email protected]; [email protected]Search for more papers by this authorAbstract
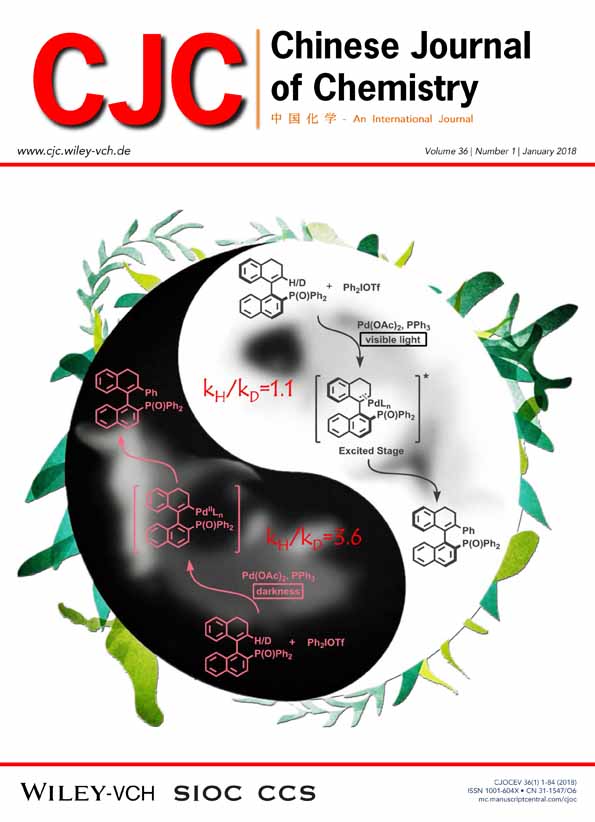
A visible light accelerated C–H functionalization reaction in palladium-catalyzed arylation of vinyl arenes with diaryliodonium salts is reported in the absence of additional photosensitizer. The kinetic isotope effect (kH/kD) was changed from 3.6 (under darkness) to 1.1 when irradiated by visible light, which indicated that the C–H functionalization step was the rate determining step under darkness and significantly accelerated by the irradiation of visible light. Finally the synthesis of ortho tetra-substituted vinylarene atropisomers with high enantiospecificity was realized via this protocol.
Supporting Information
| Filename | Description |
|---|---|
| cjoc201700618-sup-0001-AppendixS1.pdfPDF document, 14.2 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Falck, J. R.; Bejot, R.; Barma, D. K.; Bandyopadhyay, A.; Joseph, S.; Mioskowski, C. J. Org. Chem. 2006, 71, 8178.
- 2Brachet, E.; Hamze, A.; Peyrat, J.-F.; Brion, J.-D.; Alami, M. Org. Lett. 2010, 12, 4042.
- 3(a) Nakamura, M.; Fujimoto, T.; Endo, K.; Nakamura, E. Org. Lett. 2004, 6, 4837; (b) Carson, M. W.; Giese, M. W.; Coghlan, M. J. Org. Lett. 2008, 10, 2701; (c) Shi, Y.; Peterson, S. M.; Haberaecker III, W. W.; Blum, S. A. J. Am. Chem. Soc. 2008, 130, 2168; (d) Suarez, L. L.; Greaney, M. F. Chem. Commun. 2011, 47, 7992; (e) Vercruysse, S.; Cornelissen, L.; Nahra, F.; Collard, L.; Riant, O. Chem. Eur. J. 2014, 20, 1834; (f) Itoh, T.; Shimizu, Y.; Kanai, M. J. Am. Chem. Soc. 2016, 138, 7528.
- 4Dai, J.; Wang, M.; Chai, G.; Fu, C.; Ma, S. J. Am. Chem. Soc. 2016, 138, 2532.
- 5Paek, S.-M. Molecules 2012, 17, 3348.
- 6(a) Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem. Int. Ed. 2012, 51, 10236; (b) Rouquet, G.; Chatani, N. Angew. Chem. Int. Ed. 2013, 52, 11726; (c) Cao, C.; Wang, W.; Zhang, F.; Huang, N.; Zou, K. Chin. J. Chem. 2015, 33, 1077; (d) Liu, Y.; Dong, W. Chin. J. Chem. 2017, 35, 1491; (e) Liu, W.; Zheng, X.; Zeng, J.; Cheng, P. chin. J. Org. Chem. 2017, 37, 1.
- 7(a) Hatamoto, Y.; Sakaguchi, S.; Ishii, Y. Org. Lett. 2004, 6, 4623; (b) Tsujita, H.; Ura, Y.; Matsuki, S.; Wada, K.; Mitsudo, T.; Kondo, T. Angew. Chem. Int. Ed. 2007, 46, 5160; (c) Xu, Y.-H.; Lu, J.; Loh, T.-P. J. Am. Chem. Soc. 2009, 131, 1372; (d) Yang, Y.; Cheng, K.; Zhang, Y. Org. Lett. 2009, 11, 5606; (e) Yu, H.; Jin, W.; Sun, C.; Chen, J.; Du, W.; He, S.; Yu, Z. Angew. Chem. Int. Ed. 2010, 49, 5792; (f) Zhang, Y.; Cui, Z.; Li, Z.; Liu, Z.-Q. Org. Lett. 2012, 14, 1838; (g) Chen, M.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. Angew. Chem. Int. Ed. 2013, 52, 14196; (h) Wu, P.; Wang, L.; Wu, K.; Yu, Z. Org. Lett. 2015, 17, 868.
- 8(a) Martinez, R.; Chevalier, R.; Darses, S.; Genet, J.-P. Angew. Chem. Int. Ed. 2006, 45, 8232; (b) Colby, D. A.; Bergman, R. G.; Ellman, J. A. J. Am. Chem. Soc. 2006, 128, 5604; (c) Yotphan, S.; Bergman, R. G.; Ellman, J. A. J. Am. Chem. Soc. 2008, 130, 2452; (d) Xu, Y.-H.; Lu, J.; Loh, T.-P. J. Am. Chem. Soc. 2009, 131, 1372; (e) Besset, T.; Kuhl, N.; Patureau, F. W.; Glorius, F. Chem. Eur. J. 2011, 17, 7167; (f) Ano, Y.; Tobisu, M.; Chatani, N. Org. Lett. 2012, 14, 354; (g) Wang, D.; Wang, F.; Song, G.; Li, X. Angew. Chem. Int. Ed. 2012, 51, 12348; (h) Boultadakis-Arapinis, M.; Hopkinson, M. N.; Glorius, F. Org. Lett. 2014, 16, 1630; (i) Gu, Q.; Al Mamari, H. H.; Graczyk, K.; Diers, E.; Ackermann, K. Angew. Chem. Int. Ed. 2014, 53, 3868; (j) Shang, R.; Ilies, L.; Asako, S.; Nakamura, E. J. Am. Chem. Soc. 2014, 136, 14349; (k) Hu, X.-H.; Zhang, J.; Yang, X.-F.; Xu, Y.-H.; Loh, T.-P. J. Am. Chem. Soc. 2015, 137, 3169; (l) Lin, C.; Li, D.; Wang, B.; Yao, J.; Zhang, Y. Org. Lett. 2015, 17, 1328; (m) Kong, W.-J.; Liu, Y.-J.; Xu, H.; Chen, Y.-Q.; Dai, H.-X.; Yu, J.-Q. J. Am. Chem. Soc. 2016, 138, 2146; (n) Caspers, L. D.; Finkbeiner, P.; Nachtshein, B. J. Chem. Eur. J. 2017, 23, 2748.
- 9(a) Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102; (b) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322; (c) Wu, L.-Z.; Chen, B.; Li, Z.-J.; Tung, C.-H. Acc. Chem. Res. 2014, 47, 2177; (d) Xuan, J.; Zhang, Z.-G.; Xiao, W.-J. Angew. Chem. Int. Ed. 2015, 54, 15632; (e) Chen, J.-C.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Acc. Chem. Res. 2016, 49, 1911; (f) Yoon, T. P. Acc. Chem. Res. 2016, 49, 2307; (g) Ghosh, I.; Marzo, L.; Das, A.; Shaikh, R.; König, B. Acc. Chem. Res. 2016, 49, 1566; (h) Margrey, K. A.; Nicewicz, D. A. Acc. Chem. Res. 2016, 49, 1997; (i) Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075; (j) Nicholls, T. P.; Leonori, D.; Bissember, A. C. Nat. Prod. Rep. 2016, 33, 1248; (k) Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898; (l) Huang, H.; Jia, K.; Chen, Y. ACS Catal. 2016, 6, 4983; (m) Zhang, L.; Meggers, E. Acc. Chem. Res. 2017, 50, 320.
- 10For a review: (a) Xie, J.; Jin, H.; Pan, X.; Zhu, C. Tetrahedron Lett. 2014, 55, 36. For some typical results: (b) Elofson, R. M.; Gadallah, F. F. J. Org. Chem. 1971, 36, 1769; (c) Condie, A. G.; González-Gómez, J. C.; Stephenson, C. R. J. J. Am. Chem. Soc. 2010, 132, 1464; (d) Kalyani, D.; McMurtrey, K. B.; Neufeldt, S. R.; Sanford, M. S. J. Am. Chem. Soc. 2011, 133, 18566; (e) To, W.-P.; Tong, G. S.-M.; Lu, W.; Ma, C.; Liu, J.; Chow, A. L.-F.; Che, C.-M. Angew. Chem. Int. Ed. 2012, 51, 2654; (f) Xie, J.; Xu, P.; Li, H.; Xue, Q.; Jin, H.; Cheng, Y.; Zhu, C. Chem. Commun. 2013, 49, 5672; (g) Jiang, H.; Cheng, Y.; Wang, R.; Zheng, M.; Zhang, Y.; Yu, S. Angew. Chem. Int. Ed. 2013, 52, 13289; (h) Fabry, D. C.; Zoller, J.; Raja, S.; Rueping, M. Angew. Chem. Int. Ed. 2014, 53, 10228; (i) Wang, L.; Wei, X.-J.; Jia, W.-L.; Zhong, J.-J.; Wu, L.-Z.; Liu, Q. Org. Lett. 2014, 16, 5842; (j) Cuthbertson, J. D.; MacMillan, D. W. C. Nature 2015, 519, 74; (k) Jeffrey, J. L.; Terrett, J. A.; MacMillan, D. W. C. Science 2015, 349, 1523; (l) Li, J.; Zhang, J.; Tan, H.; Wang, D. Z. Org. Lett. 2015, 17, 2522; (m) Kurandina, D.; Parasram, M.; Gevorgyan, V. Angew. Chem. Int. Ed. 2017, 56, 14212.
- 11For visible light and transition metal-involved coupling reactions: (a) Hopkinson, M. N.; Tlahuext-Aca, A.; Glorius, F. Acc. Chem. Res. 2016, 49, 2261; (b) Tellis, J. C.; Kelly, C. B.; Primer, D. N.; Jouffroy, M.; Patel, N. R.; Molander, G. A. Acc. Chem. Res. 2016, 49, 1429; (c) Fabry, D. C.; Rueping, M. Acc. Chem. Res. 2016, 49, 1969; (d) Gauchot, V.; Sutherland, D. R.; Lee, A.-L. Chem. Sci. 2017, 8, 2885.
- 12(a) Zou, Y.-Q.; Duan, S.-W.; Meng, X.-G.; Hu, X.-Q.; Gao, S.; Chen, J.-R.; Xiao, W.-J. Tetrahedron 2012, 68, 6914; (b) Lu, Z.; Yoon, T. P. Angew. Chem. Int. Ed. 2012, 51, 10329; (c) Xia, X.-D.; Xuan, J.; Wang, Q.; Lu, L.-Q.; Chen, J.-R.; Xiao, W.-J. Adv. Syn. Catal. 2014, 356, 2807; (d) Masuda, Y.; Ishida, N.; Murakami, M. J. Am. Chem. Soc. 2015, 137, 14063; (e) Ding, W.; Lu, L.-Q.; Zhou, Q.-Q.; Wei, Y.; Chen, J.-R.; Xiao, W.-J. J. Am. Chem. Soc. 2017, 139, 63; (f) Welin, E. R.; Le, C.; Arias-Rotondo, D. M.; McCusker, J. K.; MacMillan, D. W. C. Science 2017, 355, 380.
- 13(a) Kalyani, D.; Deprez, N. R.; Desai, L. V.; Sanford, M. S. J. Am. Chem. Soc. 2005, 127, 7330; (b) Daugulis, O.; Zaitsev, V. G. Angew. Chem. Int. Ed. 2005, 44, 4046; (c) Phipps, R. J.; Gaunt, M. J. Science 2009, 323, 1593; (d) Merritt, E. A.; Olofsson, B. Angew. Chem. Int. Ed. 2009, 48, 9052; (e) Phipps, R. J.; McMurray, L.; Ritter, S.; Duong, H. A.; Gaunt, M. J. J. Am. Chem. Soc. 2012, 134, 10773.
- 14Feng, J.; Li, B.; He, Y.; Gu, Z. Angew. Chem. Int. Ed. 2016, 55, 2186.
- 15For convenience, compound 2A was selected in kinetic studies because of its relatively lower reaction rate.
- 16The true catalytic cycle might be complicated since UV-Vis spectrum has no absorption at 530 nm, while 530 nm light was one of the most efficient light sources.
- 17(a) Crivello, J. V.; Lee, J. L. J. Polym. Sci., Part A: Polym. Chem. 1989, 27, 3951; (b) Dektar, J. L.; Hacker, N. P. J. Org. Chem. 1990, 55, 639; (c) Shirai, A.; Kubo, H.; Takahashi, E. J. Photopolym. Sci. Technol. 2002, 15, 29; (d) Neufeldt, S. R.; Sanford, M. S. Adv. Synth. Catal. 2012, 354, 3517.