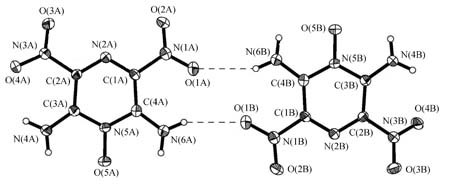
Crystal Structure, Safety Performance and Density-Functional Theoretical Investigation of 2,6-Diamino-3,5-dinitropyrazine-1-oxide (LLM-105)
Graphical Abstract
The single crystal of 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105) suitable for X-ray measurement was obtained. The bulk state of LLM-105 was also studied using density functional theory of Dmol3 code. The heat of formation for LLM-105 was evaluated and the detonation velocity (D) and detonation pressure (P) were estimated by using Kamlet-Jacobs equation. The calculation on bond dissociation energy suggests that the C-NO2 bond should be the trigger bond during the pyrolysis initiation process. The specific heat capacity (Cp) was determined and the time of the thermal decomposition from initialization to thermal explosion (adiabatic time-to-explosion) was obtained.