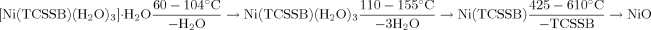Synthesis, crystal structure, properties and thermoanalysis of complexes of Cu(ll) and Ni(ll) with taurine-5-chlorosalicylaldelyde schiff base
Abstract
The reactions of transition metal salts with taurine 5-chlorosalicylaldelyde Schiff base gave two complexes [Ni(TCSSB)(H2O)3].H2O (1) and [Cu(TCSSB)(H2O)2]2[Cu(TCSSB)2].6H2O (2) (TCSSB=taurine-5-chlorosalicylaldelyde Schiff base), which were characterized by elemental analysis and X-ray diffraction analysis. The complex 1 crystallized in monoclinic system with space group P2 1/c, and a=1.4816(2) nm, b=1.3953(2) nm, c= 0.7466(1) nm, β= 100.499(3)°, V=1.5176(4) nm3, Z=4, and an infinite 3-D network structure was formed by hydrogen bonds among sulfo group, crystal water and coordinated water. Complex 2 crystallized in triclinic system with space group P1, with the cell parameters: a = 0.6413(2) nm, b= 1.4596(3) nm, c= 1.6188(4) nm, a= 102.473(5)°, β= 98.979(4)°, γ=101.739°, V=1.4165(6) nm3, Z=1. The coordination environment between Cu(1) and Cu(2) is different. Cu(1) is slightly distorted square pyramidal while Cu(2) is distorted square-plane. The complex 1 is mononuclear while the complex 2 is made up of two coordinated subunits, namely [Cu(TCSSB)2] and [CU(TCSSB)(H2O)2]2. Besides that the TG-DTG of the complex 1 was analyzed, the thermal decomposition reaction of the complex was studied under a non-isothermal condition by TG-DTG. The TG and DTG curves indicate that the complex was decomposed in three stages: