Concise and Effective Synthesis of 1→2 α-Linked Mannopyranosyl Oligosaccharides and Related Antigenic Factor 34 and Dominant of Antigenic Factor 13
Yu-Liang Zhu
Research Center for Eco- Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
Search for more papers by this authorFan-Zuo Kong
Research Center for Eco- Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
Search for more papers by this authorYu-Liang Zhu
Research Center for Eco- Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
Search for more papers by this authorFan-Zuo Kong
Research Center for Eco- Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
Search for more papers by this authorAbstract
A highly concise and effective synthesis of 1 → 2 α-linked mannopyranosyl oligosaccharides was achieved via TMSOTf promoted condensation of the corresponding benzoylated monosaccharide alkyl orthoester. 1→2 α-Linked mannosyl di, trisaccharide, antigenic factor 34, and dominant of antigenic factor 13 were readily synthesized by the new method.
References
- 1 Goodman, D. S.; Teplitz, E. D.; Wishner, A; Klein, R. S.; Burk, P. C.; Hershenbaum, E., J. Am. Acad. Dermatol. 1987, 17, 210.
- 2 Bodey, G. P., Am. J. Med. 1984, 77, 13.
- 3 Spebar, M. J.; Pruitt, B. A., J. Trauma 1981, 21, 237.
- 4 Silverman, S. Jr.; Luangjarmekorn, L; Greenspan, D., J. Oral Med. 1984, 39, 194.
- 5 Suzuki, S., Yakugaku Zasshi 1995, 115 (4), 280.
- 6 Suzuki, A; Shibata, N; Suzuki, M; Saitoh, F; Oyamada, H; Kobayashi, H; Suzuki, S; Okawa, Y., J. Biol. Chem. 1997, 272, 16822.
- 7 Shibata, N; Akagi, R; Hosoya, T; Kawahara, K; Suzuki, A; Ikuda, K; Kobayashi, H; Hisamichi, K; Okawa, Y; Suzuki, S., J. Biol. Chem. 1997, 271, 9259.
- 8 Ansaruzzaman, M; Albert, M. J.; Holme, T; Jansson, P; Rahman, M. M.; Widmalm, G., Eur. J. Biochem. 1996, 237, 786.
- 9 Nukada, T; Kitajima, T; Nakahara, Y; Ogawa, T., Carbohydr. Res. 1992, 228, 157.
- 10 Wang, W; Kong, F., J. Org. Chem. 1998, 63, 5744.
- 11
Wang, W;
Kong, F.,
Angew. Chem. Int. Ed. Engl.
1999,
38,
1247.
10.1002/(SICI)1521-3773(19990503)38:9<1247::AID-ANIE1247>3.0.CO;2-J CAS PubMed Web of Science® Google Scholar
- 12 Zhu, Y; Kong, F., Synlett 2000, 663.
- 13 Procedure for the preparation of 4 from 3: To a stirred solution of 3 (5.74 g, 10 mmol) dissolved in anhydrous dichioromethane (50 mL) TMSOTf (50 μL, 0.03 equiv) was added dropwise at - 42°C with N2 protection. The reaction mixture was stirred for 1 h, at the end of which time TLC (3/1 petroleum ether/ethyl acetate) indicated that the reaction was complete. Then the mixture was neutralized with triethylamine, concentrated under reduced pressure to dryness. Further purification by column chromatography (3/1 petroleum ether/ethyl acetate) gave 4 (3.79 g, 66%) as a colorless solid.
- 14 Lindhorst, T. K., J. Carbohydr. Chem. 1997, 16, 237.
- 15 Schmidt, R. R.; Kinzy, W., Adv. Carbohydr. Chem. Biochem. 1994, 50, 21.
- 16 Wang, W; Kong, F., Carbohydr. Res. 1999, 315, 128.
- 17 Kobayashi, H; Tanaka, S; Suzuki, J; Kiuchi, Y; Shibata, N; Suzuki, S; Okawa Y., FEMS Microbiology Leu. 1997, 152, 235.
- 18
All new compounds gave satisfactory elemental analysis. Selected physical data and 1H NMR (400 MHz, CDCl3) data are as follows: For 4: m.p. 148–150°C, [α]
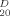 + 3.0° (c 1.3, CHCl3); 1H NMR δ: 8.07–7.33 (m, 30H, ArH), 5.99 (t, J = 9.9 Hz, 1H), 5.90–5.85 (m, 4H), 5.70 (dd, J =1.7, 3.0 Hz, 1H, H-2′), 5.28–5.18 (m, 2H, CH2 = CHCH2), 5.16 (d, J=1.5 Hz, 1H), 5.11 (d, J = 1.7 Hz, 1H), 4.61–4.47 (m, 5H), 4.42–4.39 (m, 1H), 4.37 (dd, J=1.5, 2.9 Hz, 1H), 4.15–4.13 (m 1H), 3.95–3.92 (m, 1H), 2.03 (s, 3H, CH3CO). Anal. Calcd. for C59H52O18: C 67.56, H 4.96; Found: C 67.60, H4.92. For 16: m.p. 140–143°C, [α]
+ 3.0° (c 1.3, CHCl3); 1H NMR δ: 8.07–7.33 (m, 30H, ArH), 5.99 (t, J = 9.9 Hz, 1H), 5.90–5.85 (m, 4H), 5.70 (dd, J =1.7, 3.0 Hz, 1H, H-2′), 5.28–5.18 (m, 2H, CH2 = CHCH2), 5.16 (d, J=1.5 Hz, 1H), 5.11 (d, J = 1.7 Hz, 1H), 4.61–4.47 (m, 5H), 4.42–4.39 (m, 1H), 4.37 (dd, J=1.5, 2.9 Hz, 1H), 4.15–4.13 (m 1H), 3.95–3.92 (m, 1H), 2.03 (s, 3H, CH3CO). Anal. Calcd. for C59H52O18: C 67.56, H 4.96; Found: C 67.60, H4.92. For 16: m.p. 140–143°C, [α]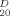 -8.4° (c 1.1, CHCl3); 1H NMR δ: 8. 14–7.25 (m, 65H, ArH), 6.35 (t, J= 10.0 Hz, 1H), 6.02 (t, J = 9.9Hz, 1H), 5.92–5.74 (m, 8H), 5.62 (dd, J = 9.9, 3.2 Hz, 1H), 5.58 (dd, J = 3.1, 1.5 Hz, 1H), 5.48 (d, 1H,), 5.30 (dd, 1H, CH2 = CHCH2), 5.27 (s, 1H), 5.22 (dd, 1H, CH2=CHCH2), 5.13 (d, J= 1.5 Hz, 1H), 5.10(s, 1H), 5.03 (s, 1H), 4.81–4.75 (m, 2H), 4.71 (dd, J = 3.1. 1.5 Hz, 1H), 4.60–4.40 (m, 8H), 4.47–4.42 (m. 2H), 4.27–4.15 (m, 4H), 3.93–3.85 (m. 2H), 3.82–3.78 (m. 1H), 2.52–3.48 (m, 1H). 2.22 (s, 3H. CH3CO), 2.06 (s, 3H. CH3CO), 2.04 (s, 3H. CH3CO). Anal. Calcd. for C130H114O42: C 66.50. H4.86; Found: C 66.55, H4.82. For 17: m.p. 136–139°C, [α]
-8.4° (c 1.1, CHCl3); 1H NMR δ: 8. 14–7.25 (m, 65H, ArH), 6.35 (t, J= 10.0 Hz, 1H), 6.02 (t, J = 9.9Hz, 1H), 5.92–5.74 (m, 8H), 5.62 (dd, J = 9.9, 3.2 Hz, 1H), 5.58 (dd, J = 3.1, 1.5 Hz, 1H), 5.48 (d, 1H,), 5.30 (dd, 1H, CH2 = CHCH2), 5.27 (s, 1H), 5.22 (dd, 1H, CH2=CHCH2), 5.13 (d, J= 1.5 Hz, 1H), 5.10(s, 1H), 5.03 (s, 1H), 4.81–4.75 (m, 2H), 4.71 (dd, J = 3.1. 1.5 Hz, 1H), 4.60–4.40 (m, 8H), 4.47–4.42 (m. 2H), 4.27–4.15 (m, 4H), 3.93–3.85 (m. 2H), 3.82–3.78 (m. 1H), 2.52–3.48 (m, 1H). 2.22 (s, 3H. CH3CO), 2.06 (s, 3H. CH3CO), 2.04 (s, 3H. CH3CO). Anal. Calcd. for C130H114O42: C 66.50. H4.86; Found: C 66.55, H4.82. For 17: m.p. 136–139°C, [α]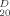 −9.9°(c 1.2, CHCl3); 1H NMR δ: 8.00–7.25 (m. 60H. ArH), 6.15 (t, J = 9.8 Hz, 1H), 5.97 (t, J = 9.8 Hz. 1H). 5.90–5.82 (m, 6H), 5.79 (dd, J = 9.8, 3.0 Hz, 1H,), 5.70 (dd, J = 9.9, 3.1Hz, 1H), 5.65 (dd, J = 3.0, 1.5 Hz, 1H), 5.49 (dd, J = 3.0, 1.5 Hz, 1H), 5.36 (s, 1H), 5.32 (s, 1H), 5.26 (dd, J=17.2, 1.4 Hz, 1H, CH2 = CHCH2), 5.20 (s, 1H), 5.18 (dd, J = 10. 3, 1.4 Hz, 1H, CH2 = CHCH2), 5.08 (d, J= 1.6 Hz, 1H), 4.80 (s, 1H), 4.69–4.37 (m, 18H), 4.24 (dd, J = 3.0, 1.5 Hz, 1H), 4.17–4.12 (m, 1H), 3.79–3.75 (m, 1H), 2.14 (s, 3H, CH3CO), 1.99 (s,6H, 2CH3CO), 1.97 (s, 3H, CH3CO). Anal. Calcd. for C125H112O42: C 65.67, H 4.90; Found: C 65.64, H 4.93.
−9.9°(c 1.2, CHCl3); 1H NMR δ: 8.00–7.25 (m. 60H. ArH), 6.15 (t, J = 9.8 Hz, 1H), 5.97 (t, J = 9.8 Hz. 1H). 5.90–5.82 (m, 6H), 5.79 (dd, J = 9.8, 3.0 Hz, 1H,), 5.70 (dd, J = 9.9, 3.1Hz, 1H), 5.65 (dd, J = 3.0, 1.5 Hz, 1H), 5.49 (dd, J = 3.0, 1.5 Hz, 1H), 5.36 (s, 1H), 5.32 (s, 1H), 5.26 (dd, J=17.2, 1.4 Hz, 1H, CH2 = CHCH2), 5.20 (s, 1H), 5.18 (dd, J = 10. 3, 1.4 Hz, 1H, CH2 = CHCH2), 5.08 (d, J= 1.6 Hz, 1H), 4.80 (s, 1H), 4.69–4.37 (m, 18H), 4.24 (dd, J = 3.0, 1.5 Hz, 1H), 4.17–4.12 (m, 1H), 3.79–3.75 (m, 1H), 2.14 (s, 3H, CH3CO), 1.99 (s,6H, 2CH3CO), 1.97 (s, 3H, CH3CO). Anal. Calcd. for C125H112O42: C 65.67, H 4.90; Found: C 65.64, H 4.93.




