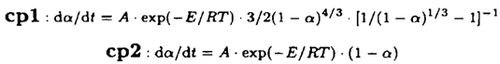Studies on synthesis and kinetics of thermodecomposition of the nickel(II) compounds with Schiff base derived from amino acids
Abstract
Monoaqua salicylaldehyde-o-aminobenzoic acid Ni(II) monohydrate (cpl) and monoaqua o-vanillin-o-aminobenzoic acid Ni(II) monohydrate (cp2) were synthesized. The composition and structures of these two compounds were analyzed. Their thermal stability and non-isothermal kinetics were also investigated by use of TG and DTG curves. The possible reaction mechanisms in their first steps of thermal decomposition reactions were deduced by means of integral and differential methods. Thermodecomposition kinetic equations of the compounds are as follows: