Dupilumab treatment of a case of asthma, chronic rhinosinusitis, and atopic dermatitis after initial benralizumab administration
Graphical Abstract
The present paper reports a patient with asthma, chronic rhinosinusitis, and atopic dermatitis treated by dupilumab after initial benralizumab administration. Benralizumab was moderately effective on asthma but not for chronic rhinosinusitis and atopic dermatitis. Dupilumab was strongly effective on both asthma and atopic dermatitis but not on chronic rhinosinusitis.
Dear Editor,
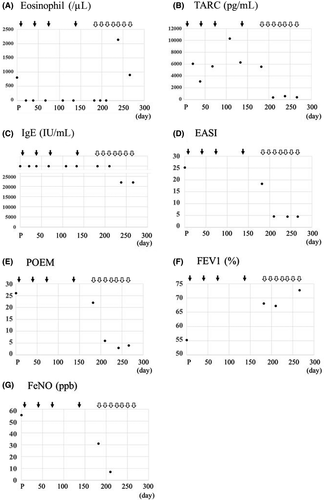
A 36-year-old Japanese man with a 16 year history of asthma and chronic rhinosinusitis (CRS), and a 7 year history of atopic dermatitis (AD) was treated with benralizumab. Because of poor control for AD, dupilumab was started at 7 weeks after the 4th application of benralizumab (Figure 1). As shown in Figure 1, after starting benralizumab, there was rapid disappearance of eosinophils in peripheral blood, but there was neither change nor substantial improvement in the eczema area and severity index (EASI), serum levels of IgE and thymus, activation-regulated chemokine (TARC), or patient-oriented eczema measure (POEM). Moreover, although there was improvement of the percent predicted forced expiratory volume in one second (FEV1%) and the fractional exhaled nitric oxide (FeNO), these did not reach the normal range. After starting dupilumab, all parameters, with the exception for increased eosinophil counts, rapidly improved and returned to the normal range. As for CRS, neither benralizumab nor dupilumab rendered any beneficial effects on the size of the polyp (data not shown).
Consistent with the significant therapeutic effects of benralizumab seen in asthma patients, especially for those with higher peripheral eosinophilic counts,1, 2 asthma improved during benralizumab administration in the present case. In addition, administration of dupilumab following the benralizumab resulted in further therapeutic effects on the asthma, which is consistent with previously reported therapeutic effects for dupilumab in asthma patients.3 Moreover, the combination effects of benralizumab and dupilumab cannot be excluded, as there were no eosinophils detected at the start of dupilumab administration. Clinical trials examining the effects of biologics targeting interleukin (IL)-5 have reported achieving no success in AD,4 while the effects of benralizumab on AD have yet to be examined. Accordingly, although peripheral eosinophils were completely removed during the benralizumab administration, the present case showed no significant improvement in the AD. In contrast, dupilumab had significant therapeutic effects on AD, which was consistent with previous studies.5-7 In addition, current results support the report that serum TARC is a disease-specific marker of AD.8 Biologics targeting IL-5 have been shown to have some therapeutic effects in approximately 60% of CRS patients.9 Furthermore, dupilumab has been reported to exhibit significant therapeutic effects on CRS.10 However, in the present case, during the administration of both benralizumab and dupilumab, no significant effects on CRS were detected with the exception of improvement in subjective symptoms. Meanwhile, as the FeNO value might reflect the status of not only asthma but also CRS, the possibility of therapeutic effects associated with benralizumab and dupilumab on CRS cannot be ruled out in the present case. The current results might also suggest that the effects of biologics (ie, benralizumab and dupilumab) by themselves are occasionally limited with regard to CRS, especially for the polyp size.
Demonstration of the effects of benralizumab and dupilumab on asthma, CRS, and AD in the same patient, such as the present case, is of benefit with regard to examining the pathogenesis of each disease. The current results also suggest that IL-4Rα signaling is strongly involved in the pathogenesis of asthma and AD.
CONFLICT OF INTEREST
None declared.