Synthesis and Biological Evaluation of a Spongistatin AB-Spiroketal Analogue.
Abstract
For Abstract see ChemInform Abstract in Full Text.
ChemInform Abstract
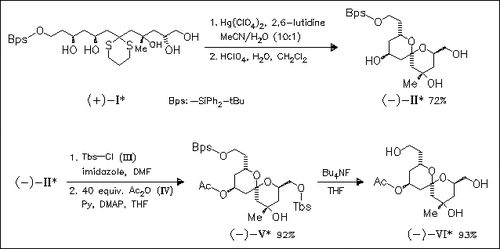
The synthesis of the spongistatin AB-spiroketal mimic (VI) is carried out with dithianepentanol (I), derived via a one-pot unsymmetrical linchpin dithiane coupling of epoxides. Dithiane removal and acid treatment afford spiroketal (II), which is selectively silylated and acetylated to give, after deprotection, the title analogue (VI). Cytotoxic data on this compound, as well as those of a recently described parent spongistatin congener reveal that neither (VI) nor its parent compound are active against several human cancer cell lines. Moreover, no effects of these compounds are observed in an antitubulin activity test.