Stereoselective Synthesis of Cycloheptanone Derivatives via an Intermolecular [5 + 2] Cycloaddition Reaction.
Abstract
For Abstract see ChemInform Abstract in Full Text.
ChemInform Abstract
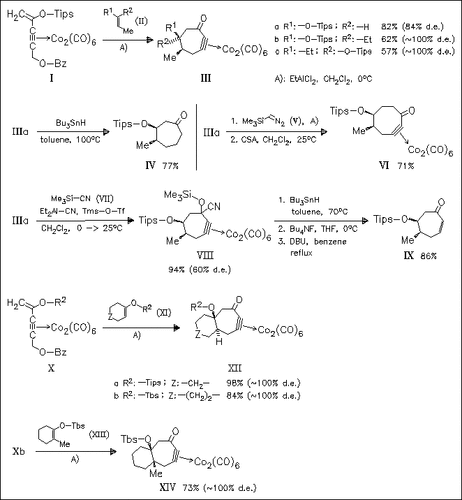
The [5 + 2] cycloaddition reaction of a dicobalt hexacarbonyl propargyl cation unit with various silyl enol ethers in the presence of EtAlCl2 provides a novel access towards cycloheptanone derivatives. The seven-membered cyclic cobalt-masked acetylenic adducts can be decomplexed with Bu3SnH or serve as suitable educts for further transformations. The cycloaddition reaction proceeds with remarkable diastereoselectivity. cis-Substituted adducts (III) are obtained from acyclic silyl enol ethers irrespective of the double bond geometry. Reaction of cyclic silyl enol ethers provides cis- or trans-fused bicyclic adducts depending on the degree of substitution at the double bond [cf. (XII) and (XIV)].