ChemInform Abstract: Synthesis, Antiviral and Cytostatic Activities, of Carbocyclic Nucleosides Incorporating a Modified Cyclopentane Ring. Part 4. Adenosine and Uridine Analogues.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
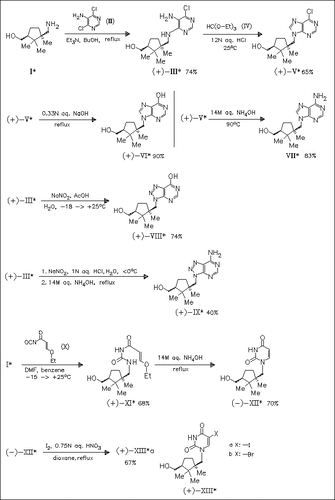
New carbocyclic nucleosides are prepared by mounting a purine [cf. (V), (VI), (VII)], 8-azapurine [cf. (VIII), (IX)] or pyrimidine [cf. (XII), (XIII)] on the amino group of the aminoalcohol (I). The products are evaluated as antiviral and antitumor agents. Only purine derivative (V) displays any cytostatic activity.